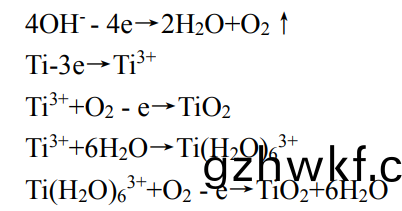前言(yan)
钛在地(di)球(qiu)上(shang)的(de)储量(liang)十(shi)分丰(feng)富,在(zai)金属元(yuan)素(su)中位(wei)列第(di)七(qi)�。以(yi)钛为(wei)主要元素(su)的(de)钛(tai)合金(jin)有(you)很(hen)多(duo)优点(dian),其(qi)中以TC4钛(tai)合金(jin)用(yong)途(tu)最(zui)广,而(er)且(qie)相(xiang)对(dui)于(yu)其(qi)它合金(jin)来说(shuo)�,因其具有(you)质(zhi)量(liang)轻�����、耐(nai)腐(fu)蚀性好(hao)、比强度(du)高、弹性(xing)模(mo)量低(di)、生(sheng)物(wu)相容性(xing)好等被广泛(fan)应用(yong)于(yu)航空航天[1]、海洋船(chuan)舶[2]、生物医药(yao)[3]等领域(yu)����。然而(er)钛合金本身的(de)耐腐蚀性(xing)较差(cha)�����,尤其是在(zai)海洋环(huan)境(jing)的(de)恶(e)劣(lie)条(tiao)件(jian)下,海水中含(han)有大量(liang)的氧化(hua)性Cl-��,使其(qi)比(bi)普通(tong)水(shui)环境更(geng)具(ju)腐蚀性�����,纯(chun)钛合金的(de)防腐(fu)性(xing)能(neng)达(da)不到(dao)所(suo)需(xu)的要求��,这(zhe)使(shi)得钛(tai)及其合(he)金零部件在(zai)高盐(yan)��、高(gao)湿的(de)海洋环(huan)境(jing)中(zhong)工作时,会(hui)遭受严重的电化学(xue)腐(fu)蚀(shi)和缝(feng)隙腐蚀,极大(da)的(de)限制了它的大(da)规模(mo)应用(yong)[4]。虽然依靠(kao)其(qi)表(biao)面(mian)自身(shen)的(de)氧(yang)化膜,钛(tai)合(he)金(jin)具(ju)有(you)一(yi)定的(de)耐(nai)腐(fu)蚀(shi)性,但(dan)自(zi)然(ran)形(xing)成的(de)氧(yang)化(hua)膜较(jiao)薄(bao)��,易(yi)被(bei)破坏(huai)���,导(dao)致其耐(nai)腐蚀(shi)性(xing)能(neng)有限。为(wei)了(le)增强其(qi)耐(nai)腐蚀(shi)性,提(ti)高在(zai)海洋环(huan)境中的(de)使(shi)用(yong)寿命���,钛(tai)合(he)金表(biao)面处(chu)理是(shi)一种(zhong)有(you)效(xiao)的(de)方法(fa)�。近(jin)些年来发展(zhan)起来的(de)微(wei)弧氧化技术(Micro-arcoxidation,MAO)因其绿(lv)色(se)环(huan)保、操(cao)作简单、膜(mo)层(ceng)与(yu)基体(ti)结合(he)力(li)强(qiang)且(qie)膜层(ceng)致密的优点而被(bei)广(guang)泛(fan)应(ying)用于(yu)镁(mei)�����、铝�、钛等(deng)金(jin)属(shu)表面处(chu)理中(zhong)[5-7]。
微(wei)弧(hu)氧化(hua)技(ji)术(shu)可(ke)以在钛(tai)合金表面(mian)生(sheng)成(cheng)可(ke)控(kong)的致密(mi)氧(yang)化陶瓷(ci)膜����,以减(jian)缓(huan)腐(fu)蚀(shi)。但(dan)是(shi)微(wei)弧(hu)氧(yang)化处理(li)会在(zai)钛合(he)金基(ji)体表面留(liu)下较多(duo)的孔洞及(ji)裂(lie)纹(wen)�,抑制(zhi)了其(qi)耐(nai)腐(fu)蚀性能的(de)进(jin)一步(bu)提高。近(jin)年(nian)来,许(xu)多(duo)学(xue)者通过(guo)封孔(kong)方法来(lai)提(ti)高(gao)微弧氧化膜(mo)的(de)耐(nai)腐蚀(shi)性(xing)��,包括在微弧氧化(hua)电(dian)解(jie)液中加(jia)入(ru)不溶性(xing)的(de)微纳(na)米粒(li)子(zi):氧(yang)化石(shi)墨烯(xi)[8-10]���、氮化(hua)硼(peng)[11-13]��、碳(tan)纳米(mi)管[14-16]�����、碳(tan)化(hua)硅[17-19]氮化硅(gui)[20-22]����、聚四(si)氟(fu)乙(yi)烯(xi)[23-24]�����、氧(yang)化锌(xin)[25-26]等进行(xing)原(yuan)位(wei)封孔����,或者通(tong)过(guo)微弧氧(yang)化(hua)技(ji)术与(yu)其(qi)它表(biao)面(mian)处(chu)理(li)技术相结合的(de)后封(feng)孔方法(fa)。BA等[27]采(cai)用微弧(hu)氧(yang)化(hua)与(yu)水(shui)热生(sheng)长(zhang)相(xiang)结合的方(fang)法(fa),在微(wei)弧(hu)氧化(hua)后的(de)镁合金表面原位(wei)生(sheng)长插有(you)肉豆(dou)蔻(kou)酸(suan)离子的水(shui)滑(hua)石(shi)膜(mo)����,微(wei)弧(hu)氧化膜中(zhong)的微(wei)孔被水(shui)滑石封闭(bi),表(biao)面(mian)变得疏水��,复(fu)合涂层(ceng)最(zui)低(di)腐(fu)蚀电(dian)流(liu)密(mi)度(du)较基体降(jiang)低了(le)5个(ge)数(shu)量(liang)级�����,明显提高(gao)了(le)镁(mei)合金的(de)耐(nai)腐蚀性(xing)能(neng)。于(yu)浩(hao)洋(yang)等(deng)[28]采用(yong)微(wei)弧氧(yang)化和(he)溶(rong)胶(jiao)凝胶(jiao)结(jie)合的(de)方(fang)法(fa)�,在(zai)NiTi合(he)金表面(mian)先(xian)掺(can)杂ZnO微粒进行微(wei)弧(hu)氧(yang)化(hua),后利(li)用(yong)聚(ju)丙烯酰(xian)胺(an)溶(rong)胶凝胶进(jin)行封(feng)孔(kong)处理(li),提高了(le)NiTi合(he)金的(de)耐腐(fu)蚀(shi)性(xing)��,且(qie)在潮湿的(de)条件(jian)下(xia)具有一定(ding)的(de)自(zi)修复(fu)能(neng)力��。莫(mo)格等(deng)[29]在微(wei)弧氧化(hua)后的(de)镁(mei)合金上涂装聚苯胺改性(xing)的环(huan)氧(yang)树(shu)脂(zhi)��,所(suo)制(zhi)得(de)的(de)复(fu)合(he)涂层(ceng)腐蚀电流(liu)密(mi)度下降了(le)3个数(shu)量(liang)级(ji)����,显(xian)著(zhu)提(ti)高了复(fu)合(he)涂层(ceng)对镁(mei)合金(jin)基(ji)体的腐蚀防护(hu)能力(li)。HE等(deng)[30]结合微(wei)弧氧(yang)化和激光(guang)加(jia)工技术在S355海(hai)洋钢表面(mian)制(zhi)备(bei)了(le)复(fu)合(he)涂层,该(gai)复合(he)涂层(ceng)与基体(ti)层(ceng)结合良好�����,具(ju)有(you)良好的(de)力(li)学(xue)性能��;当(dang)微弧(hu)氧化(hua)的(de)电(dian)流密度(du)为(wei)5A.dm−2时,复(fu)合(he)涂层的耐(nai)腐蚀(shi)性最好(hao)�,腐蚀(shi)电流(liu)降(jiang)低(di)了3个(ge)数量级(ji)。
受(shou)自(zi)然(ran)界启(qi)发(fa),研究者(zhe)发(fa)现在材料表(biao)面构(gou)建(jian)超疏(shu)水(shui)膜可以减少(shao)水溶(rong)性(xing)腐蚀(shi)介(jie)质与基体(ti)的接(jie)触���,从而(er)减(jian)少(shao)腐蚀[31]。周(zhou)垲(kai)杰(jie)等(deng)[32]在(zai)镁(mei)合(he)金(jin)表(biao)面(mian)涂(tu)覆(fu)环氧树脂粘(zhan)结层(ceng),再(zai)向(xiang)其喷涂微(wei)米(mi)二(er)氧(yang)化(hua)硅(gui)颗粒和(he)纳(na)米聚四氟乙烯(xi)颗粒(li)构(gou)建(jian)超(chao)疏水(shui)膜,其具有(you)优异的耐腐蚀(shi)性能(neng)以及自清洁、防污(wu)特(te)性(xing)。杨(yang)文广(guang)等[33]在铝合(he)金表面(mian)制备镁铝(lv)水(shui)滑(hua)石膜,并(bing)用(yong)全(quan)氟辛(xin)基三(san)乙氧(yang)基硅(gui)烷(wan)修饰����,制(zhi)备出(chu)耐腐(fu)蚀(shi)性(xing)能优(you)异的超(chao)疏(shu)水膜�。很多(duo)研究者采(cai)用(yong)微弧(hu)氧(yang)化技术(shu)结(jie)合(he)低表面能(neng)物质自(zi)组装在(zai)镁(mei)����、铝(lv)合(he)金表(biao)面(mian)构建(jian)超(chao)疏(shu)水膜来提高其(qi)耐腐(fu)蚀(shi)性(xing)能(neng)[34-35],但在(zai)钛(tai)合(he)金(jin)上(shang)的相关研(yan)究(jiu)较(jiao)少。基于(yu)此�,本研(yan)究(jiu)采(cai)用(yong)微弧(hu)氧化技(ji)术����,在(zai)TC4钛(tai)合金(jin)表(biao)面(mian)构(gou)建微纳(na)米(mi)结(jie)构,并采(cai)用十(shi)八(ba)烷(wan)基三(san)甲氧(yang)基(ji)硅烷(Octadecyltrimethoxysilane,OTMS)进行(xing)表(biao)面(mian)修(xiu)饰,既(ji)对微弧氧(yang)化膜层起(qi)到封孔作用(yong)��,又赋予(yu)其低(di)表(biao)面能,从而(er)在其表面(mian)形(xing)成(cheng)了一层长效耐(nai)久的抗腐蚀超疏(shu)水膜��,有效提(ti)高了(le)钛(tai)合金的(de)耐腐蚀性能(neng)�����,在钛(tai)合金表(biao)面(mian)防(fang)护(hu)方面具有广(guang)阔(kuo)的应(ying)用前(qian)景。
1、试(shi)验(yan)
1.1钛(tai)合金(jin)预处(chu)理
TC4钛合金(jin)购(gou)自(zi)东莞市(shi)宏迪(di)金(jin)属(shu)材料有(you)限(xian)公司(si)����,其主(zhu)要(yao)成(cheng)分(fen)(质量分数)为(wei)6%Al、4%V、0.3%Fe、0.01%C、0.03%O,其余为(wei)Ti。将直(zhi)径为15mm����、厚度为(wei)1.5mm的(de)圆形试样(yang)依(yi)次(ci)用800、1000、1500、2000目SiC砂纸(zhi)打磨����,去除表(biao)面(mian)氧化膜��,并(bing)用(yong)无(wu)水乙(yi)醇和(he)去离子水(shui)分(fen)别超(chao)声(sheng)清(qing)洗10min,自然(ran)晾(liang)干。
1.2超疏水(shui)微弧氧化(hua)膜的制备(bei)
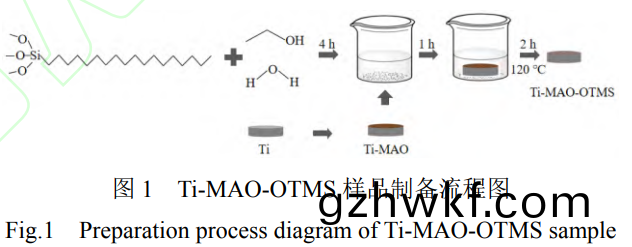
将预处(chu)理后(hou)的TC4钛(tai)合金试样放入3L的(de)10g/L九(jiu)水偏(pian)硅酸钠����、10g/L十(shi)二(er)水磷酸三(san)钠和2g/L氢(qing)氧化(hua)钾(jia)的混合(he)溶(rong)液中进(jin)行(xing)微弧(hu)氧(yang)化(hua)。采用(yong)直(zhi)流脉冲电源(yuan)�����,试(shi)样(yang)接(jie)电(dian)源(yuan)正极,不(bu)锈(xiu)钢电解液(ye)槽(cao)接(jie)电源(yuan)负(fu)极,具体(ti)工(gong)艺参(can)数(shu)为(wei):恒流模式(shi)�,电流密(mi)度(du)为(wei)18A/dm2,脉(mai)冲(chong)频(pin)率为500Hz,占空(kong)比为(wei)20%,氧(yang)化(hua)时(shi)间(jian)为20min��。使用机械(xie)搅拌和(he)循环(huan)冷(leng)却(que)装(zhuang)置(zhi)保(bao)持电解(jie)液温(wen)度在(zai)50℃以(yi)下�����。微(wei)弧(hu)氧(yang)化试验完成后,取(qu)出样(yang)品����,并(bing)用(yong)去(qu)离子(zi)水冲(chong)洗(xi),晾(liang)干(gan)备(bei)用��,命(ming)名为Ti-MAO。
将微(wei)弧(hu)氧化后(hou)的样品(pin)在(zai)室温下浸(jin)入OTMS乙(yi)醇(chun)溶液(ye)(OTMS、去(qu)离子水�、乙(yi)醇按1:1:18的(de)体(ti)积比(bi)配(pei)制(zhi)����,静(jing)置4h)中(zhong)1h����,取出后(hou)在(zai)120℃下(xia)固(gu)化(hua)2h,制备流程如(ru)图1所(suo)示(shi),所(suo)得(de)样品(pin)命名(ming)为
Ti-MAO-OTMS。作(zuo)为(wei)对照,将(jiang)预(yu)处(chu)理的钛(tai)合(he)金(jin)试样(yang)直接(jie)浸入OTMS溶(rong)液中(zhong)1h并在同(tong)样(yang)条件下(xia)固化(hua),命(ming)名为(wei)Ti-OTMS。
1.3性能表(biao)征(zheng)
采用扫(sao)描(miao)电子(zi)显微镜(SEM����,JSM-IT100��,JEOL)对样品(pin)表面(mian)形貌(mao)进行观察(cha)����。并(bing)利(li)用所配备的(de)能(neng)量分散谱(pu)仪(yi)(EDS)确(que)定(ding)样品表面(mian)成分��。使(shi)用(yong)傅(fu)立叶(ye)变(bian)换红外(wai)衰减(jian)全(quan)反(fan)射(she)光(guang)谱(pu)仪(yi)(FTIRATR,NicoletIS1,ThermoFisher)对(dui)样(yang)品(pin)的(de)官(guan)能(neng)团进(jin)行(xing)分析(xi)�����。通过(guo)X射线(xian)粉末(mo)衍(yan)射(she)仪(yi)(XRD�,MAX2500VB3+/PC,Rigaku)分(fen)析微(wei)弧(hu)氧(yang)化膜(mo)的(de)结(jie)构(gou)����。使用(yong)接(jie)触(chu)角(jiao)测量仪(JC2000D1,POWEREACH)表征(zheng)涂层的(de)润湿性��。每(mei)次(ci)使用5μL的水滴����,在不(bu)同(tong)位置(zhi)至(zhi)少重(zhong)复5次(ci)�,取(qu)其(qi)平(ping)均(jun)值�。利(li)用光学(xue)轮(lun)廓仪(ContourGTK0)测(ce)试样(yang)品(pin)表面(mian)粗糙度(du)(Ra),测(ce)试(shi)范围为(wei)241μm×180.8μm。
采(cai)用(yong)电化学站(zhan)(CHI660E,武(wu)汉(han)辰华)进(jin)行电化学(xue)测(ce)试��。所有试验(yan)均采用常规的(de)三(san)电极电化学(xue)方法(fa)进行(xing)��,其(qi)中(zhong)饱(bao)和甘(gan)汞(gong)电(dian)极(ji)作为参比电(dian)极����,铂(bo)电(dian)极(ji)作为(wei)对(dui)电(dian)极,试(shi)样作为(wei)工作电(dian)极(ji)�。用(yong)硅橡胶密(mi)封(feng)试(shi)样,留(liu)下(xia)10mm×10mm的(de)面(mian)积。在(zai)测(ce)试(shi)之(zhi)前(qian),钛合金(jin)试样(yang)在3.5wt%的NaCl溶液(ye)中浸(jin)泡(pao)30min����。电化(hua)学测试主(zhu)要包括动电(dian)位(wei)极(ji)化(hua)和(he)电化(hua)学(xue)阻抗(kang)测量(liang)����。扫(sao)描速(su)率为5mV/s,电位(wei)范(fan)围(wei)为-1~1V进(jin)行动(dong)电位极化(hua)�����。同(tong)时使用塔菲尔(er)外推法计算(suan)了(le)腐(fu)蚀(shi)电(dian)流密度(du)(icorr)、腐(fu)蚀(shi)电(dian)位(wei)(Ecorr)并计算(suan)出(chu)腐(fu)蚀(shi)速(su)率(Pi),在(zai)0.01Hz和(he)100kHz之间(jian)进(jin)行(xing)电化(hua)学(xue)阻抗(kang)测(ce)量,并用Z-View软件对(dui)测(ce)试结果(guo)进(jin)行(xing)拟合(he)。
2�����、结(jie)果与(yu)讨论
2.1表(biao)面形(xing)貌和组(zu)成(cheng)分(fen)析(xi)
图2为(wei)不同(tong)样(yang)品(pin)的SEM图。从图2a中可(ke)以看出,钛合金(jin)基(ji)体(ti)经过SiC砂(sha)纸打磨(mo)后�����,表(biao)面平(ping)整��,其(qi)表面(mian)Ra值(zhi)仅为0.28μm。图(tu)2b中(zhong),钛合金经微弧(hu)氧化(hua)后表(biao)面(mian)出现(xian)很多(duo)微(wei)凸起(qi)及小的(de)裂缝,微凸(tu)起尺寸(cun)在(zai)几到(dao)几十(shi)微(wei)米(mi)不等�����,Ra值(zhi)增(zeng)大到(dao)5.09μm�。这主(zhu)要(yao)是因为MAO反(fan)应(ying)过程(cheng)中的(de)火(huo)花放(fang)电阶(jie)段(duan)会在钛(tai)合金(jin)表面形成大(da)量的(de)放电通道,熔(rong)融态(tai)的(de)氧化(hua)物以(yi)类(lei)似火山喷(pen)发(fa)的方(fang)式从(cong)这(zhe)些通(tong)道中(zhong)大(da)量喷溅(jian)而(er)出(chu),遇到(dao)温度低(di)的电解(jie)液骤冷形(xing)成氧化物(wu)陶(tao)瓷(ci)膜(mo)[36]�����。由(you)于放电(dian)顺序(xu)的不(bu)同(tong),MAO涂层表面呈现连续(xu)多(duo)孔(kong)的(de)岛(dao)状(zhuang)分(fen)布(bu)�����。从图(tu)2b中Ti-MAO的(de)高(gao)放(fang)大倍数(shu)SEM图(tu)可以(yi)明显(xian)看(kan)出(chu)�����,钛合(he)金(jin)经微弧(hu)氧化后表面(mian)出现了(le)纳(na)米(mi)级的(de)小凸(tu)起及(ji)放(fang)电(dian)通(tong)道留下的(de)孔洞�,为超(chao)疏水(shui)膜的构(gou)建(jian)提(ti)供了(le)可(ke)能。从(cong)图(tu)2c可以看(kan)出(chu),Ti-MAO-OTMS表面(mian)形貌(mao)没(mei)有(you)明显变(bian)化(hua),但是(shi)其(qi)表面裂缝明(ming)显减(jian)少(shao),部分孔(kong)洞(dong)被(bei)填充(chong),而(er)且其Ra值降低(di)到(dao)3.86μm�����,这(zhe)可(ke)能(neng)是(shi)因(yin)为(wei)OTMS可(ke)以(yi)较好(hao)的填充(chong)进微弧(hu)氧(yang)化(hua)的裂(lie)缝及(ji)孔(kong)洞(dong)进行封孔�。

Ti-MAO的(de)FT-IR谱(pu)图中1080cm-1处(chu)的(de)峰对应于(yu)硅氧键(jian)的伸缩振动峰(feng)(图3)�,来源于(yu)溶(rong)液中(zhong)的(de)硅酸盐转化(hua)的SiO2���。相(xiang)比(bi)于(yu)Ti-MAO,Ti-MAO-OTMS的FT-IR谱(pu)图中(zhong)新出现(xian)的(de)2920cm-1和(he)2850cm-1处(chu)的峰分别对应(ying)亚甲基(ji)基团的(de)非对称(cheng)振动峰(feng)和对(dui)称伸(shen)缩振动峰(feng),进(jin)一(yi)步证明(ming)了OTMS的成功(gong)引(yin)入����。
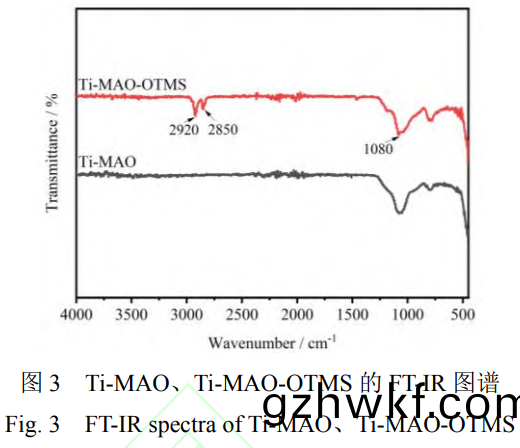
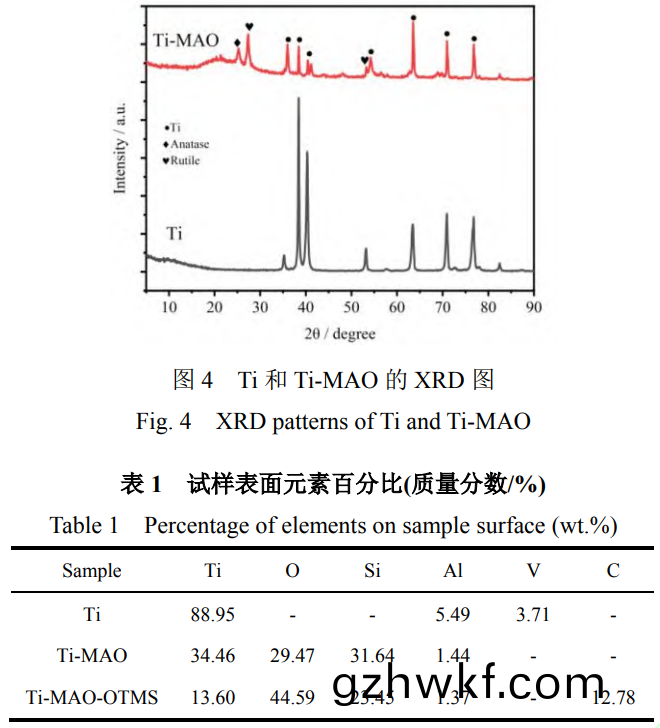
图4为(wei)钛(tai)合(he)金(jin)微弧(hu)氧化前后(hou)的XRD图(tu)。可以(yi)看(kan)出(chu)���,在(zai)25.5°和(he)27.6°处(chu)分别为(wei)TiO2的锐(rui)钛矿相(101)和金红(hong)石(shi)相(110)的衍射(she)峰(feng)�。Ti-MAO中(zhong)的(de)15°-35°
范(fan)围(wei)内(nei)出现(xian)了(le)宽峰(feng)值(zhi),说(shuo)明涂(tu)层(ceng)中(zhong)有一些(xie)非晶(jing)相(xiang)存在(zai)。结(jie)合表(biao)1中(zhong)试(shi)样(yang)表面(mian)元(yuan)素质(zhi)量百分(fen)比可知�,纯(chun)钛合(he)金(jin)基体主要以(yi)Ti元(yuan)素(su)为主��,其中夹(jia)杂着少(shao)量(liang)的(de)Al��、V等元素。而Ti-MAO样品中Ti元(yuan)素(su)含(han)量明显减(jian)少(shao),O����、Si元(yuan)素(su)含量(liang)明(ming)显增(zeng)加(jia)�,结(jie)合图(tu)3中(zhong)Ti-MAO的(de)FT-IR特征(zheng)峰(feng)�,表(biao)明(ming)该宽(kuan)峰为(wei)非晶(jing)相(xiang)的SiO2的衍射。在(zai)反应的进(jin)行中(zhong)�,电解(jie)液(ye)中(zhong)的SiO32-在电(dian)场的作(zuo)用下(xia)向阳(yang)极(ji)方(fang)向移(yi)动(dong)����,并(bing)转(zhuan)化(hua)为(wei)SiO2。电(dian)解(jie)质(zhi)的迅(xun)速冷却能(neng)够(gou)使涂层以(yi)较(jiao)高(gao)的冷却速(su)率(lv)生长(zhang),其中(zhong)SiO2以非晶(jing)形(xing)式存(cun)在于涂层(ceng)中(zhong)[37]���。说(shuo)明(ming)TC4钛(tai)合(he)金经(jing)过微(wei)弧氧(yang)化后(hou)主(zhu)要由(you)Ti�����、锐(rui)钛矿TiO2�����、金(jin)红石TiO2和(he)非晶相的SiO2组成,TiO2相主(zhu)要(yao)源于基(ji)体(ti)中(zhong)的Ti在(zai)微弧氧化过程中的氧化。将(jiang)TC4钛(tai)合(he)金作(zuo)为阳(yang)极(ji)置(zhi)于(yu)微弧(hu)氧(yang)化(hua)电解液中连通电源时,两(liang)极之间产生(sheng)电(dian)场,电解液(ye)中(zhong)的(de)阴(yin)离(li)子(zi)在(zai)电(dian)场(chang)的作(zuo)用(yong)下(xia)向(xiang)阳(yang)极(ji)迁移(yi)�,阳离(li)子(zi)向阴极(ji)迁移����,到达(da)两极(ji)表面(mian)之(zhi)后通(tong)过得失(shi)电(dian)子(zi)主要(yao)发生(sheng)如(ru)下电化(hua)学反应(ying):
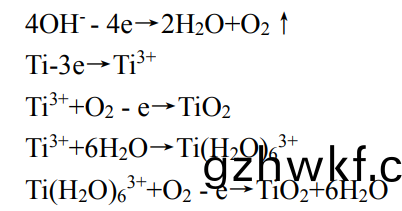
2.2表(biao)面润湿(shi)性(xing)能(neng)分析(xi)
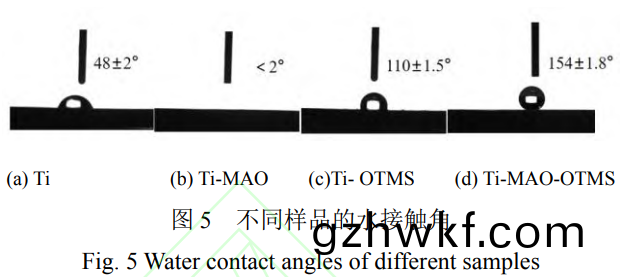
从图(tu)5a可(ke)知(zhi)�����,Ti的接触(chu)角(jiao)为48°±2°�����,表(biao)现出(chu)亲(qin)水(shui)性。经微(wei)弧(hu)氧(yang)化(hua)处理后Ti-MAO的(de)接(jie)触(chu)角(jiao)小(xiao)于(yu)2°(图5b),表(biao)现出超亲水性(xing)��。这主(zhu)要归(gui)因(yin)于微(wei)弧(hu)氧(yang)化(hua)之(zhi)后表面(mian)出现(xian)的(de)微(wei)纳(na)米粗糙(cao)结构(gou)�。图(tu)5c中Ti-OTMS的接触(chu)角为(wei)110°±1.5°��,说明(ming)其(qi)具(ju)有(you)疏(shu)水性��。而Ti-MAO-OTMS的(de)接(jie)触(chu)角为154°±1.8°(图(tu)5d)����,而(er)且其滚(gun)动(dong)角(jiao)约为3°±1°,表现(xian)出(chu)超疏水(shui)性。这主(zhu)要归因(yin)于(yu)钛合(he)金(jin)基体表(biao)面(mian)微(wei)纳米结(jie)构的(de)构建(jian)和低(di)表面能(neng)物(wu)质(zhi)OTMS的(de)成功(gong)修(xiu)饰(shi)。疏(shu)水(shui)性的提(ti)高有利于改(gai)善其耐腐(fu)蚀(shi)性。
2.3防(fang)腐性(xing)能(neng)分析
从(cong)图(tu)6a的(de)Bode阻(zu)抗图可(ke)以(yi)看出(chu)Ti-MAO-OTMS涂层(ceng)低频下的(de)阻(zu)抗(kang)模量(|Hz0.01|)比Ti和(he)Ti-MAO高了(le)约两个数量(liang)级(ji),达(da)到(dao)4.97×107Ω���。结合(he)图(tu)6b中(zhong)Tafel极化(hua)曲(qu)线和表(biao)2所(suo)示的(de)拟(ni)合(he)电(dian)参(can)数,可以(yi)看出相比(bi)于Ti,Ti-MAO的(de)Ecorr提(ti)升(sheng)了0.416V,icorr降低了(le)约2个(ge)数量级(ji)���,达(da)到了6.893×10-8A·cm-2。这归因(yin)于微弧氧化膜内(nei)部(bu)致(zhi)密(mi)层的阻(zu)挡作(zuo)用(yong)。而(er)经过(guo)OTMS修饰(shi)的(de)Ti-MAO-OTMS的(de)Ecorr提高了(le)0.934V����,达到(dao)0.454V���;icorr降低(di)了近4个(ge)数量(liang)级,达(da)到(dao)了9.481×10-10A·cm-2。经过超(chao)疏(shu)水(shui)膜层(ceng)修(xiu)饰后(hou)����,其Pi较(jiao)纯(chun)钛(tai)合金降低(di)了3个数(shu)量级(ji)��。图(tu)6c为(wei)不(bu)同样(yang)品的Nyquist图。一(yi)般来说�,Nyquist图(tu)中(zhong)容抗(kang)弧(hu)直(zhi)径(jing)越(yue)大(da),说明膜的(de)耐蚀(shi)性(xing)能(neng)越好[38]。可(ke)以(yi)看(kan)出(chu)��,钛合(he)金基体Ti容抗(kang)弧直径最(zui)小(xiao)�,具有(you)最(zui)差(cha)的耐(nai)腐(fu)蚀(shi)性。
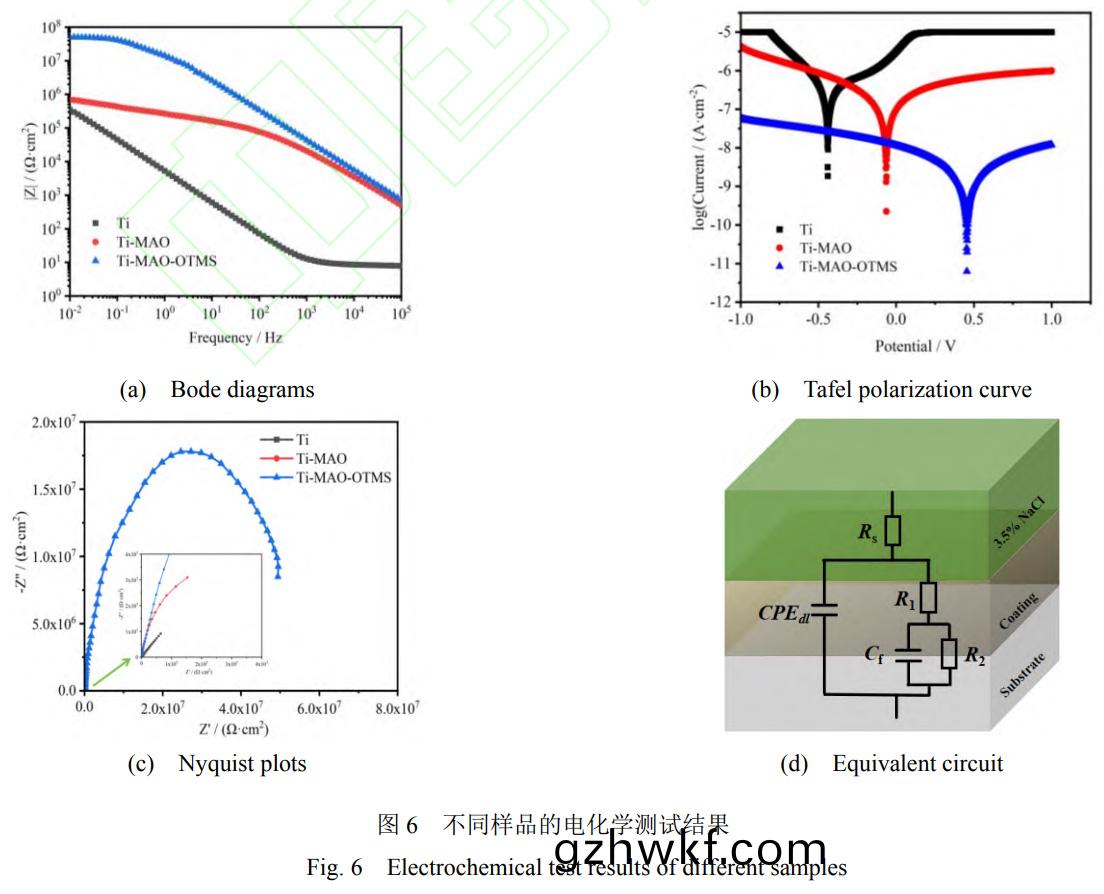
Ti-MAO样(yang)品的容抗弧直径有所(suo)增大(da)�,说明(ming)其(qi)耐腐(fu)蚀性(xing)有(you)所(suo)提高(gao)。由(you)于(yu)Ti-MAO表面(mian)较多(duo)的(de)微(wei)孔(kong)和(he)裂纹有利(li)于(yu)腐(fu)蚀(shi)性离(li)子的(de)渗(shen)入(ru)�,使其耐(nai)腐蚀(shi)性提(ti)高(gao)有(you)限。而Ti-MAO-OTMS样(yang)品(pin)的(de)容抗弧直径(jing)明显(xian)进(jin)一步增(zeng)大��,说明其具有(you)最(zui)佳的(de)耐腐(fu)蚀性(xing)。这归因(yin)于(yu)其优异(yi)的(de)超疏水(shui)性(xing)能(neng),有(you)效阻挡(dang)了Cl-等腐蚀性(xing)物(wu)质(zhi)对基体造(zao)成(cheng)的(de)损(sun)伤��。为了进一步(bu)分析(xi)样品在(zai)3.5wt%NaCl溶(rong)液中(zhong)的电(dian)化学性能(neng)�����,使用(yong)图(tu)6d所示的等效电路拟(ni)合(he)电化(hua)学(xue)阻(zu)抗(kang)谱�����,具体(ti)参(can)数(shu)如(ru)表(biao)3所示。Rs为(wei)溶(rong)液电阻(zu)�,R1和(he)CPEdl分别表示电(dian)解(jie)质(zhi)溶液(ye)与膜层/基(ji)底之间(jian)的(de)电阻和(he)双电(dian)层(ceng)电(dian)容(rong)。R2和Cf分别为电阻(zu)和膜层在(zai)表面的(de)电容。R1值(zhi)越(yue)高,耐腐(fu)蚀性(xing)越好。
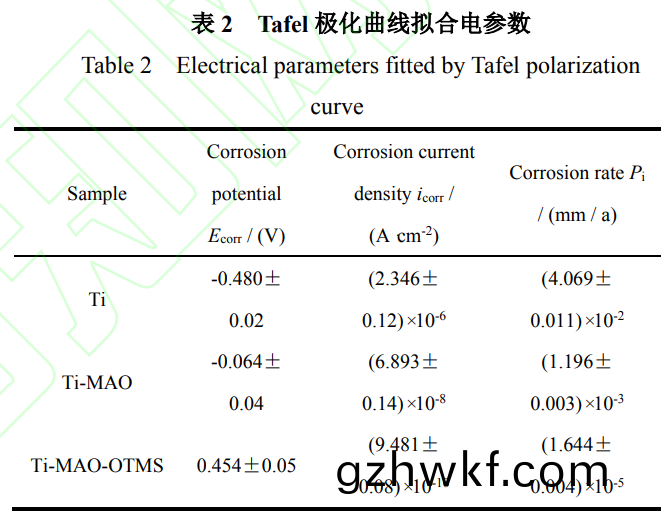
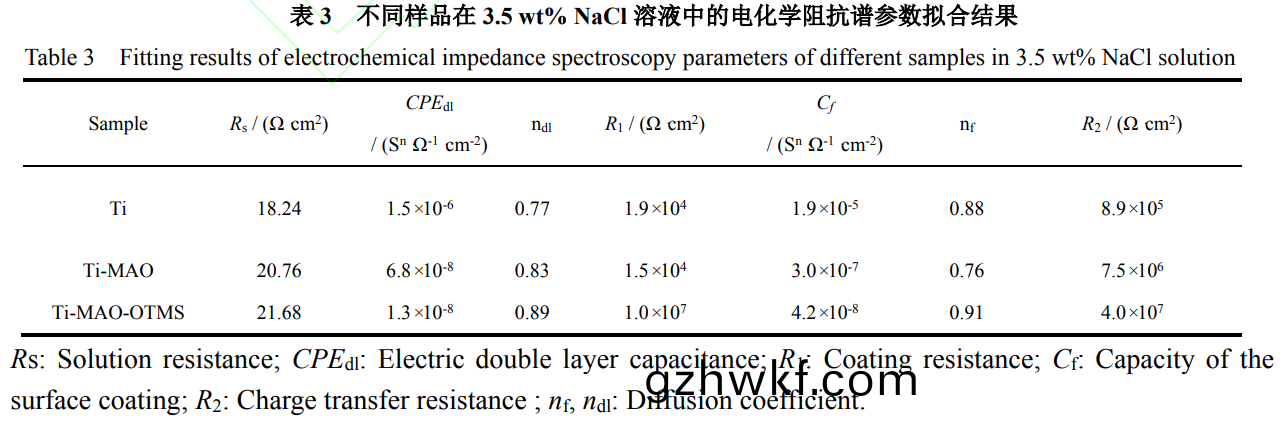
由(you)表(biao)3中的数(shu)据(ju)可(ke)知,样品(pin)Ti的(de)R2远大于(yu)R1���,说(shuo)明钛(tai)合(he)金基(ji)体的(de)耐蚀(shi)性主要由钝(dun)化膜决定。样(yang)品Ti-MAO的(de)R1相对于Ti的(de)R1有所降(jiang)低��,这(zhe)是(shi)由于(yu)微弧(hu)氧化(hua)后试样表面(mian)变成超(chao)亲水(shui)�����,腐(fu)蚀溶液(ye)中的(de)腐蚀介质更(geng)容易(yi)与(yu)试样表面接(jie)触(chu)���,更(geng)容(rong)易(yi)完成吸附过程。然而(er)�,Ti-MAO的(de)膜(mo)层(ceng)电阻R2比基(ji)体增大(da)近(jin)10倍,这证(zheng)明了微(wei)弧氧(yang)化后TiO2膜层(ceng)内(nei)部(bu)密度更(geng)大�����,使得(de)微弧氧化(hua)样(yang)品的(de)表(biao)面(mian)耐(nai)腐(fu)蚀(shi)性优(you)于(yu)未经处理的(de)基(ji)体(ti)样(yang)品。微弧(hu)氧(yang)化后(hou)的(de)样(yang)品(pin)经低(di)表面能(neng)物(wu)质(zhi)修(xiu)饰(shi)后(hou)���,样(yang)品(pin)表(biao)面(mian)由超(chao)亲(qin)水(shui)性变(bian)为(wei)超(chao)疏(shu)水(shui)性(xing),Ti-MAO-OTMS的R2相对(dui)于(yu)基(ji)体增大(da)将近(jin)50倍����。由于(yu)膜(mo)层(ceng)的超疏水(shui)性(xing)���,R1比(bi)未(wei)处理(li)的基体提高(gao)了(le)约500倍(bei),比微(wei)弧(hu)氧(yang)化后的样(yang)品提(ti)高约(yue)700倍(bei)����。因为超(chao)疏水(shui)表面(mian)将腐蚀介质(zhi)与(yu)样品表(biao)面分(fen)开(kai)���,使得超(chao)疏(shu)水试(shi)样(yang)表(biao)面的(de)耐蚀性(xing)优(you)于基(ji)体和(he)仅微弧(hu)氧(yang)化处理(li)后(hou)的试样(yang)����。通过以上电化(hua)学分(fen)析可(ke)以看出���,与(yu)纯(chun)钛合金基体(ti)相(xiang)比,钛合金(jin)微(wei)弧(hu)氧(yang)化(hua)结(jie)合(he)OTMS修(xiu)饰(shi)得(de)到的复合膜明(ming)显提高(gao)了基(ji)体的耐蚀(shi)性(xing),且防腐效(xiao)果显(xian)著(zhu)优于单(dan)一的微弧(hu)氧(yang)化膜(mo)。
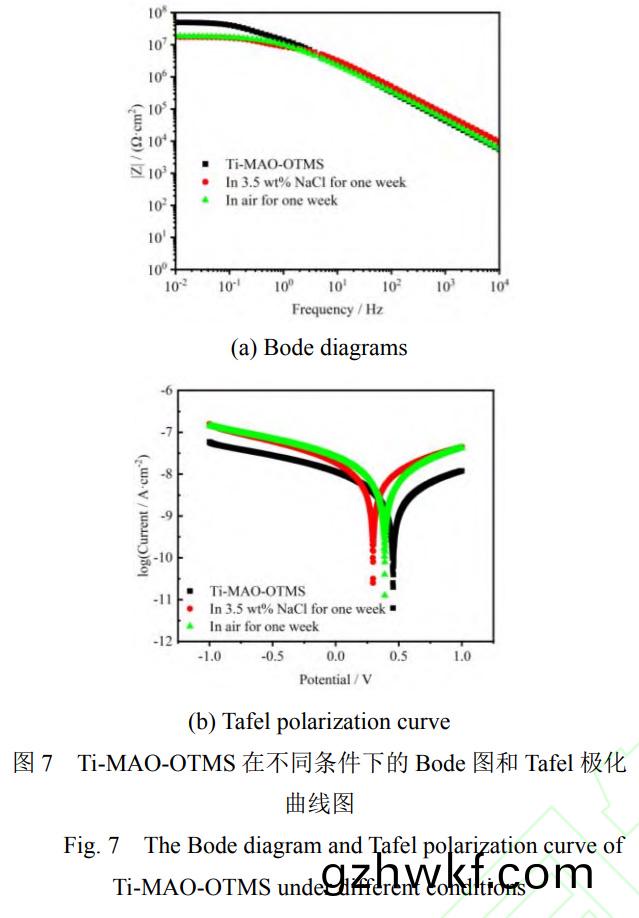
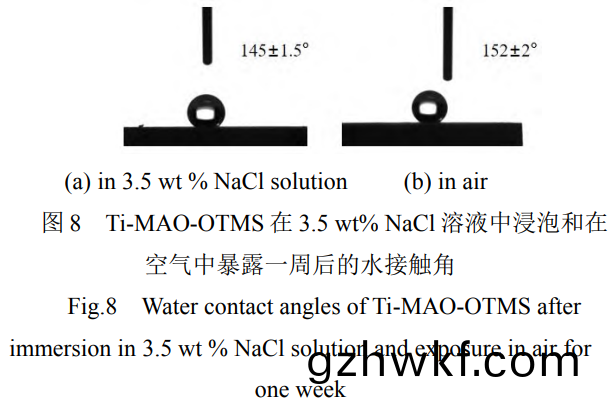
研究了(le)Ti-MAO-OTMS样(yang)品的(de)腐蚀耐(nai)久性,结果如图(tu)(7a�、7b)所示���。可以(yi)看出(chu)����,Ti-MAO-OTMS分别在3.5wt%NaCl溶液中(zhong)浸(jin)泡和(he)在空气中暴露(lu)一(yi)周(zhou)后(hou),其阻抗值略(lve)有(you)下(xia)降,但仍都(dou)能达到(dao)107数(shu)量级(ji)。在(zai)3.5wt%NaCl溶(rong)液(ye)中浸泡一(yi)周(zhou)后其阻抗(kang)值(zhi)略有下(xia)降(jiang)�����,但(dan)仍(reng)都(dou)能(neng)达(da)到107数(shu)量级����。在3.5wt%NaCl溶(rong)液(ye)中浸泡(pao)一(yi)周后,样品的(de)Ecorr为(wei)0.296V,icorr仍能达(da)到(dao)2.555×10-9A·cm-2��,而(er)且(qie)其(qi)水接触角(jiao)变(bian)为(wei)145°±1.5°����,仍(reng)然具(ju)有较强的(de)疏(shu)水性(图8a)�����;室(shi)温(wen)下在空气(qi)中暴露一(yi)周后(hou),空(kong)气(qi)中(zhong)的水分(fen)��、氧气及腐蚀性介质(zhi)的(de)联合(he)作(zuo)用(yong)会引起(qi)膜的(de)破坏,导(dao)致其耐(nai)腐(fu)蚀性能(neng)有所下降,样品的Ecorr为(wei)0.388V,icorr为(wei)2.698×10-9A·cm-2,仍然具(ju)有(you)优异(yi)的(de)耐腐(fu)蚀性(xing),而(er)且(qie)其表(biao)面仍(reng)然(ran)具有超疏水(shui)性(xing)能,其(qi)水(shui)接触角(jiao)为152°±2°(图(tu)8b)。表明其具有优异的(de)腐(fu)蚀(shi)耐久性�����。
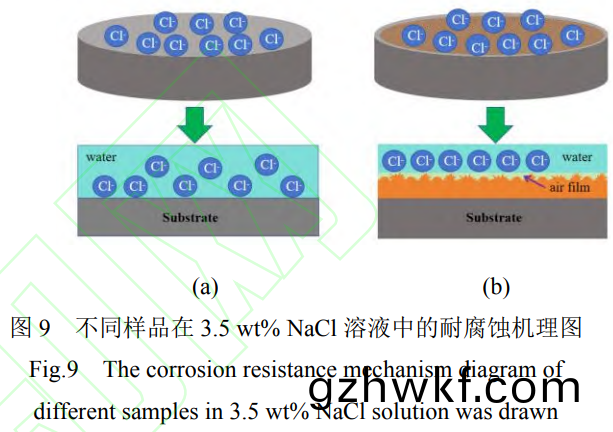
图(tu)9所示(shi)为(wei)钛(tai)合金表(biao)面超疏(shu)水(shui)膜(mo)的耐(nai)腐蚀机理示(shi)意(yi)图(tu)����。图(tu)9a为(wei)钛合(he)金基(ji)体(ti)直接(jie)与(yu)NaCl溶液(ye)接触�����,由(you)于(yu)表面只(zhi)有一(yi)层薄(bao)的(de)天(tian)然氧化膜且是(shi)亲水(shui)的����,使(shi)腐(fu)蚀(shi)性液(ye)体很容(rong)易吸(xi)附在样(yang)品(pin)表(biao)面,对样(yang)品(pin)造成破坏(huai)。而图(tu)9b中的Ti-MAO-OTMS表面(mian)获得了低(di)表(biao)面(mian)能的(de)微(wei)纳粗糙结构��,可以(yi)捕获空(kong)气(qi)并(bing)在样(yang)品(pin)表面形(xing)成空(kong)气层����,使其(qi)具(ju)有(you)超疏(shu)水性(xing)。大大(da)减(jian)小(xiao)了腐蚀性(xing)的(de)Cl-等与(yu)试样(yang)表面(mian)的接触(chu),延(yan)缓和(he)减少(shao)了(le)Cl-等在(zai)试样表(biao)面(mian)的吸(xi)附(fu)过程(cheng)��,从而(er)提(ti)高了钛(tai)合(he)金(jin)在含腐(fu)蚀性Cl-等溶液(ye)中的(de)耐(nai)腐(fu)蚀(shi)���。
3�����、结论
(1)通过(guo)微弧氧化技术在(zai)TC4钛(tai)合金(jin)表(biao)面构建了(le)以锐(rui)钛(tai)矿(kuang)和(he)金(jin)红石TiO2为主(zhu)要(yao)成分(fen)的(de)陶(tao)瓷膜(mo),阻挡了(le)水(shui)和(he)腐蚀(shi)性离子(zi)的侵入��,使(shi)钛(tai)合(he)金(jin)的(de)耐腐蚀性能有(you)所改善(shan)�����。与钛合金(jin)基(ji)体(ti)相比,其(qi)腐(fu)蚀(shi)速率下(xia)降(jiang)了1个数(shu)量(liang)级(ji),自(zi)腐(fu)蚀(shi)电流密度(du)下降了(le)2个数量(liang)级,自(zi)腐(fu)蚀(shi)电(dian)压(ya)正(zheng)移0.416V�。
(2)将(jiang)微弧(hu)氧化(hua)技(ji)术和(he)低(di)表(biao)面能(neng)物质(zhi)OTMS表面(mian)修饰相结(jie)合(he)����,在(zai)TC4钛(tai)合金表(biao)面(mian)构建(jian)了具(ju)有微(wei)纳(na)米(mi)级(ji)粗糙(cao)结构的超疏水(shui)膜(mo)层,水(shui)和(he)腐蚀性(xing)离子(zi)难以(yi)侵入基(ji)底��,从而(er)使钛合金(jin)具(ju)有(you)优异(yi)的(de)长效耐腐(fu)蚀(shi)性(xing)能。与钛(tai)合金基(ji)体(ti)相(xiang)比�,其(qi)腐蚀(shi)电(dian)流密度降低(di)了(le)近(jin)4个(ge)数(shu)量级(ji),自腐蚀电压(ya)正移(yi)动(dong)0.934V,且在(zai)3.5wt%的NaCl溶(rong)液(ye)中浸泡一周(zhou)和(he)在(zai)空(kong)气中(zhong)暴露一(yi)周后���,自(zi)腐(fu)蚀电(dian)流(liu)密度(du)仍(reng)能分别(bie)达到(dao)2.555×10-9A.cm-2和2.698×10-9A.cm-2�����。
参考(kao)文献(xian)
[1] 王欣, 罗(luo)学昆(kun), 宇波���, 等(deng). 航(hang)空(kong)航(hang)天用(yong)钛(tai)合金表面 工(gong)程技(ji)术(shu)研究(jiu)进展[J]. 航(hang)空制(zhi)造(zao)技术, 2022,65(04):
14-24. WANG Xin, LUO Xuekun, YU Bo, et al. Research progress on surface engineering technology of titanium alloy for aerospace[J]. Aeronautical Manufacturing Technology, 2022, 65 (04): 14-24. (in Chinese)
[2] 李永华(hua)����, 张(zhang)文旭, 陈(chen)小龙(long),等(deng). 海洋工程(cheng)用(yong)钛合金(jin)研(yan) 究(jiu)与(yu)应用(yong)现状[J]. 钛工(gong)业(ye)进展(zhan)���,2022�,39(01):43-48.
LI Yonghua, ZHANG Wenxu, CHEN Xiaolong, et al. Research and application status of titanium alloys for marine engineering[J]. Titanium Industry Progress, 2022, 39 (01): 43-48. (in Chinese)
[3] 廖赞(zan), 缪(mou)卫(wei)东���, 马(ma)嘉丽. 钛合(he)金(jin)在(zai)生(sheng)物医(yi)药领域应用(yong) 现状(zhuang)和(he)展(zhan)望(wang)[J]. 新(xin)材(cai)料(liao)产业(ye)�����, 2017�����,(03):19-24.
LIAO Zan, MIU Weidong, MA Jiali. Application status and prospect of titanium alloy in biomedical field[J]. Advanced Materials Industry, 2017, (03): 19-24. (in Chinese)
[4] CHEN Xiaowen, HU Jie, ZHANG Defen, et al. Study on corrosion resistance of TC4 titanium alloy micro‐arc oxidation/(PTFE+graphite) composite coating[J]. International Journal of Applied Ceramic Technology, 2022, 19(1): 397-408.
[5] 王东(dong), 刘(liu)金(jin)玉, 孙(sun)世(shi)博(bo), 等(deng). 镁合金表面(mian)微(wei)弧(hu)氧化(hua)/自组装/镍(nie)复(fu)合涂层的(de)腐(fu)蚀过(guo)程和机理(li)[J]. 中国表(biao)面工 程(cheng)�����, 2024�����,37(01):100-109.
WANG Dong, LIU Jinyu, SUN Shibo, et al. Corrosion process and mechanism of micro-arc oxidation /self-assembly/nickel composite coating on magnesium alloy surface[J]. China Surface Engineering, 2024, 37 (01): 100-109. (in Chinese)
[6] 刘(liu)磊, 李(li)来(lai)时(shi), 吴玉(yu)胜(sheng), 等(deng). 不(bu)同铝合(he)金基(ji)体黑(hei)色(se) 微弧(hu)氧(yang)化膜的厚度对(dui)其结(jie)构(gou)和性(xing)能(neng)的影(ying)响[J]. 中(zhong)国(guo)表(biao) 面工程(cheng)��, 2023,36(06):163-177.
LIU Lei, LI Laishi, WU Yusheng, et al. The effect of the thickness of black micro-arc oxidation film on the structure and properties of different aluminum alloy substrates[J]. China Surface Engineering, 2023, 36(06): 163-177. (in Chinese)
[7] 毛政, 李洪, 张津(jin)��, 等(deng). TC4 钛(tai)合金微(wei)弧(hu)氧化-溶(rong)胶(jiao) 凝胶复(fu)合涂(tu)层的(de)制备及其抗高温(wen)氧化性能(neng)[J]. 中(zhong)国(guo)表 面(mian)工程, 2015,28(03):76-81.
MAO Zheng, LI Hong, ZHANG Jin, et al. Preparation and high temperature oxidation resistance of micro-arc oxidation-sol-gel composite coating on TC4 titanium alloy[J]. China Surface Engineering, 2015, 28 (03): 76-81. (in Chinese)
[8] WANG Jing, FU Zhanghua, LIU Hao, et al. Preparation and characterization of micro-arc oxidation biological coatings on magnesium alloys containing graphene oxide[J]. Chemical Engineering Journal, 2024, 482: 149064.
[9] LI Huancai, YU Huijun, CHEN Chuanzhong, et al. Effect of graphene oxide on corrosion resistance and biological activity of micro arc oxidation ceramic layer on titanium alloy[J]. Materials Letters, 2022, 327: 133056.
[10] SHANG Wei, WU Fang, WANG Yuanyuan, et al. Corrosion resistance of micro-arc oxidation/graphene oxide composite coatings on magnesium alloys[J]. Acs Omega, 2020, 5(13): 7262-7270.
[11] CHEN Xiaowen, REN Peng, ZHANG Defen, et al. Corrosion and wear properties of h-BN-modified TC4 titanium alloy micro-arc oxide coatings[J]. Surface Innovations, 2022, 11(1-3): 49-59.
[12] LI Zhenwei, DI Shichun. The microstructure and wear resistance of microarc oxidation composite coatings containing nano-hexagonal boron nitride (HBN) particles[J]. Journal of Materials Engineering and Performance, 2017, 26: 1551-1561.
[13] GAO Yixiong, XIAO Shu, WU Hao, et al. Effect of h-BN nanoparticles incorporation on the anti-corrosion and anti-wear properties of micro-arc oxidation coatings on 2024 aluminum alloy[J]. Ceramics International, 2023, 49(23): 37475-37485.
[14] GUO Yufei, XU Luyao, Luan Junji, et al. Effect of carbon nanotubes additive on tribocorrosion performance of micro-arc oxidized coatings on Ti6Al4V alloy[J]. Surfaces and Interfaces, 2022, 28: 101626.
[15] YAZ1C1 S , MUHAFFEL F, BAYDOGAN M. Effect of incorporating carbon nanotubes into electrolyte on surface morphology of micro arc oxidized Cp-Ti[J]. Applied surface science, 2014, 318: 10-14.
[16] LIU Jiang, ZHU Xinhe, MA Dengqing, et al. Effect of nickel-coated carbon nanotubes on the preparation and wear resistance of microarc oxidation ceramic coating on ZL109 aluminum alloy[J]. Scientific Reports, 2022, 12(1): 11037.
[17] MARKOV M , PREVISLOV S , KRASIKOV A , et al. Study of the microarc oxidation of aluminum modified with silicon carbide particles[J]. Russian Journal of Applied Chemistry, 2018, 91: 543-549.
[18] DAI Ting, ZHAO Jie, YANG Xiaoyu, et al. Global and local corrosion performance of nano-SiC induced micro-arc oxidation coating on magnesium alloy[J]. Journal of Materials Engineering and Performance, 2022, 31(8): 6747-6758. [19] WANG Y Q, WANG X J, GONG W X, et al. Effect of SiC particles on microarc oxidation process of magnesium matrix composites[J]. Applied surface science, 2013, 283: 906-913.
[20] SHEN Yiding, FANG Kai, XIANG Yun, et al. Improvement in osteogenesis, vascularization, and corrosion resistance of titanium with silicon-nitride doped micro-arc oxidation coatings[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 1023032.
[21] ALIOFKHAZRAEI M, ROUHAGHDAM A , GHOBADI E. Characterization of Si3N4/TiO2 nanocomposite coatings prepared via micro arc oxidation[J]. Journal of Nanoscience and Nanotechnology, 2011, 11(10): 9057-9060.
[22] GUO Lingyun, GAO Chunna WANG Fan, et al. Influence of content of silicon nitride nanoparticles into micro-arc oxidation coating of titanium on bactericidal capability and osteoblastic differentiation[J]. Surface and Coatings Technology, 2023, 458: 129346.
[23] NIE Wenxian, XIANG Mingzhe, Yu Leiting, et al. Self-lubricating micro-arc oxidized polytetrafluoroethylene composite coating on rivet steel for improve corrosion/wear resistance[J]. Materials Chemistry and Physics, 2023, 306: 128019.
[24] CHEN Jian, LI Wangning, XU Jinxin, et al. Effect of current density and polytetrafluoroethylene on the properties of micro‐arc oxide coating of pure aluminum[J]. International Journal of Applied Ceramic Technology, 2023, 20(5): 2860-2873.
[25] Kozelskaya A , Verzunova K , Akimchenko I , et al. Antibacterial calcium phosphate coatings for biomedical applications fabricated via micro-arc oxidation[J]. Biomimetics, 2023, 8(5): 444.
[26] ZHANG Xinxin, YANG Lei, LU Xueqin, et al. Characterization and property of dual-functional Zn-incorporated TiO2 micro-arc oxidation coatings: The influence of current density[J]. Journal of Alloys and Compounds, 2019, 810: 151893.
[27] BA Zhixin, WANG Yongmin, SUN Tianyi, et al. Preparation and properties of hydrophobic micro-arc oxidation/layered double hydroxide composite coating on magnesium alloy[J]. Surface and Coatings Technology, 2023, 475: 130113.
[28] 于(yu)浩洋, 孟建(jian)兵(bing), 董(dong)小娟��, 等. NiTi 合金(jin)微(wei)弧氧(yang)化 复合(he)膜(mo)层的(de)制(zhi)备(bei)与性能[J]. 电镀与涂(tu)饰(shi)���, 2023,42(14):1-10.
YU Haoyang, MENG Jianbing, DONG Xiaojuan, et al. Preparation and properties of micro-arc oxidation composite coating on NiTi alloy[J]. Electroplating & Finishing, 2023, 42 (14): 1-10. (in Chinese)
[29] 莫格��, 崔学(xue)军, 张颖(ying)君(jun), 等. AZ31B 镁合(he)金(jin)表(biao)面微(wei) 弧(hu)氧(yang)化/聚苯胺改性环氧涂(tu)层(ceng)的腐(fu)蚀(shi)失(shi)效行(xing)为[J]. 中(zhong)国 表(biao)面工(gong)程, 2020���,33(02):37-46.
MO Ge, CUI Xuejun, ZHANG Yingjun, et al. Corrosion failure behavior of micro-arc oxidation / polyaniline modified epoxy coating on AZ31B magnesium alloy surface[J]. China Surface Engineering, 2020, 33(02): 37-46. (in Chinese)
[30] HE X, SONG R G, KONG D J. Microstructure and corrosion behaviours of composite coatings on S355 offshore steel prepared by laser cladding combined with micro-arc oxidation[J]. Applied Surface Science, 2019, 497: 143703.
[31] HUANG Niumeng, WANG Ying, ZHANG Yan, et al. Multifunctional coating on magnesium alloy: Superhydrophobic, self-healing, anti-corrosion and wear-resistant[J]. Surface and Coatings Technology, 2023, 463: 129539.
[32] 周(zhou)垲杰, 辛蕾(lei)�����, 黄(huang)小文����, 等. 镁(mei)合(he)金基底(di)超疏水涂 层的制(zhi)备及其防(fang)污(wu)防(fang)腐(fu)性能(neng)研究(jiu)[J]. 材(cai)料保(bao)护(hu)�����,2023,56(05):71-75+126. ZHOU Kaijie, XIN Lei, HUANG Xiaowen, et al. Preparation of superhydrophobic coating on magnesium alloy substrate and its antifouling and anticorrosive properties[J]. Materials Protection, 2023, 56 (05): 71-75+126. (in Chinese)
[33] 杨文(wen)广(guang)�����, 刘振(zhen)红�����, 朱梅婷(ting)�, 等. 铝合金表(biao)面超(chao)疏水(shui) 缓蚀自修(xiu)复(fu)膜的(de)制备(bei)及其耐(nai)蚀(shi)性(xing)[J]. 腐(fu)蚀(shi)与防(fang)护(hu), 2021����,42(05):1-7+37. YANG Wenguang, LIU Zhenhong, ZHU Meiting, et al. Preparation of superhydrophobic corrosion inhibition self-healing film on aluminum alloy surface and its corrosion resistance[J]. Corrosion & Protection, 2021, 42 (05): 1-7+37. (in Chinese)
[34] SHANG Wei, WANG Yuanyuan, WEN Yuqing, et al. Study on the properties of micro-arc oxidation self-assembled composite coatings on magnesium alloy[J]. International Journal of Electrochemical Science, 2017, 12(12): 11875-11891.
[35] MO Qiufeng, QIN Gemei, WEI Wu, et al. Hydrophobic composite layers for enhancing long-term corrosion resistance of Al alloy micro-arc oxidation coating[J]. Surface and Coatings Technology, 2022, 450: 128979.
[36] LIU Shimin, LI Baoe, LIANG Chunyong, et al. Formation mechanism and adhesive strength of a hydroxyapatite/TiO2 composite coating on a titanium surface prepared by micro-arc oxidation[J]. Applied Surface Science, 2016, 362: 109-114.
[37] WU Guolong, YIN Yanyi, ZHANG Shuo, et al. Effect of laser texturing on the antiwear properties of micro-arc oxidation coating formed on Ti-6Al-4V[J]. Surface and Coatings Technology, 2023, 453: 129114.
[38] WANG Ying, BAO Huayang, TANG Aiguo, et al. Ti3C2Tx-based composite coating on AZ31B Mg alloy surface for improved anti-corrosion/wear-reducing properties[J]. Materials Today Communications, 2023, 35: 105664.
作者(zhe)简介(jie):王(wang)莹(ying),女(nv),1982 年(nian)出生(sheng)��,博士,副(fu)研(yan)究(jiu)员(yuan),硕(shuo)士研(yan)究生导(dao)师����。 主(zhu)要(yao)研(yan)究(jiu)方向为(wei)功能表(biao)面(mian)与功(gong)能(neng)涂(tu)层(ceng)。 E-mail:ywang@http://www.gzhwkf.com
相关链(lian)接(jie)