钛(tai)及(ji)钛合金(jin)屈(qu)服(fu)强度(du)高(gao)�、密度(du)低�����,具有良好的力学(xue)性能,优(you)异的耐(nai)蚀(shi)性和抗(kang)冲(chong)刷性(xing)�����,[1−2]�����,广(guang)泛(fan)运用于(yu)海(hai)洋工程领(ling)域,特(te)别是海水(shui)管路(lu)和海(hai)水(shui)冷却发电装置(zhi)等[3−5]。虽(sui)然钛合金具(ju)有良好的耐蚀性,但(dan)在(zai)恶劣(lie)的(de)海(hai)洋环境中(zhong)同(tong)样(yang)面(mian)临着海(hai)水(shui)腐(fu)蚀(shi)问(wen)题����。海(hai)水腐蚀是(shi)由(you)多种(zhong)因素(su)造(zao)成的(de),包括(kuo):盐度、温(wen)度、pH以(yi)及(ji)生(sheng)物(wu)污(wu)损(sun)等(deng),其中生(sheng)物(wu)污(wu)损是最重(zhong)要的(de)因(yin)素之一(yi)[6]����。
生物(wu)污损(sun)是一(yi)个复(fu)杂的过程,涉(she)及微(wei)生(sheng)物(wu)(包括细(xi)菌����、原生(sheng)动物和微藻)和(he)大(da)型污损(sun)生物(wu)(包括藤(teng)壶(hu)、牡蛎等大型(xing)无(wu)脊椎动(dong)物和(he)大型藻(zao)类(lei))[7−8]。微(wei)生(sheng)物(wu)在(zai)材料表(biao)面(mian)附着会诱发腐蚀(MIC)���,这(zhe)已(yi)经被广泛(fan)研究[9−13]��。如(ru)钛(tai)合(he)金(jin)在流(liu)动海(hai)水(shui)中(zhong)形成的生(sheng)物薄(bao)膜(mo)约20~50µm�����,短期内对(dui)材(cai)料(liao)具(ju)有(you)保(bao)护(hu)作用(yong)[14]。Shewanella藻(zao)类(lei)通(tong)过(guo)削(xue)弱钛(tai)合(he)金(jin)钝(dun)化(hua)膜,使Cl−更(geng)具侵(qin)略(lve)性(xing)���,加剧(ju)腐蚀[15]。嗜盐(yan)菌(jun)N.tibetense在Q235钢表(biao)面非(fei)均匀局(ju)部聚(ju)集,形成(cheng)氧(yang)浓差(cha)腐蚀电(dian)池(chi)诱发局(ju)部(bu)腐(fu)蚀(shi),且N.tibetense能以(yi)Fe为能(neng)源(yuan)物(wu)质(zhi)获取电子,加(jia)速电(dian)化学反(fan)应(ying)的(de)电(dian)荷(he)转移,进(jin)而(er)加速(su)腐(fu)蚀进程�����,氧浓度差腐(fu)蚀(shi)电池(chi)和(he)N.tibetense对铁金(jin)属(shu)直接(jie)消耗(hao)两(liang)种机制耦合共(gong)同促进(jin)碳钢(gang)腐(fu)蚀[16]。细(xi)菌(jun)产生(sheng)的(de)胞外聚(ju)合(he)物质(zhi)(EPS)可(ke)以使B30铜镍合(he)金(jin)表面形成(cheng)较厚(hou)双(shuang)氧(yang)化(hua)层(ceng)(外层沉(chen)积(ji)Cu2O层(ceng)和(he)内部(bu)氧化镍层(ceng)),有效(xiao)减缓(huan)试(shi)样腐(fu)蚀速率(lv)[17]����。微生(sheng)物(wu)附(fu)着对(dui)金属(shu)的(de)腐(fu)蚀(shi)影(ying)响已(yi)经(jing)得(de)到(dao)很好的研(yan)究(jiu)�����,不(bu)同(tong)微生物的(de)生命(ming)活(huo)动(dong)对(dui)不(bu)同金(jin)属(shu)材(cai)料(liao)的腐(fu)蚀行为(wei)亦(yi)有所(suo)差(cha)异(yi)[18−21]。马(ma)士(shi)德等(deng)[22]在(zai)三(san)亚对工业(ye)纯钛(tai)(TA2)进(jin)行(xing)海(hai)水(shui)腐蚀研(yan)究(jiu)�����,结果表明致(zhi)密(mi)�����、稳(wen)定的钛(tai)氧化(hua)物膜(mo)起(qi)到了防腐作用(yong),但污(wu)损生物(wu)群落(luo)演(yan)替(ti)导致(zhi)防(fang)腐作(zuo)用(yong)难(nan)以(yi)保证(zheng)长(zhang)期(qi)性和(he)稳定性�,尤(you)其(qi)是局(ju)部环(huan)境(jing)��。同时(shi)���,已(yi)有研究表(biao)明(ming),大(da)型(xing)污损(sun)生(sheng)物(wu)短(duan)期(qi)内(nei)对不锈钢具有保(bao)护作(zuo)用(yong)��,但(dan)大型污损(sun)生(sheng)物(wu)一般(ban)是(shi)长期且不均匀(yun)附着(zhe),易(yi)在污(wu)损(sun)生物(wu)与金(jin)属(shu)材(cai)料(liao)的边(bian)缘(yuan)缝隙(xi)间形成(cheng)氧浓度(du)差(cha),从(cong)而导(dao)致(zhi)局(ju)部(bu)腐(fu)蚀[23−25]。在以往(wang)大型污(wu)损(sun)生(sheng)物研究中���,研究(jiu)材(cai)料(liao)一(yi)般为不(bu)锈(xiu)钢(gang)和(he)碳(tan)钢(gang),缺乏(fa)对钛合(he)金(jin)的(de)研究(jiu)和分析(xi)[26−27]。不(bu)同材料受(shou)海水理(li)化性质和污损生物(wu)群落(luo)演替影(ying)响(xiang)���,腐(fu)蚀行为(wei)存(cun)在差异(yi)���,且海(hai)水(shui)中(zhong)材料(liao)腐(fu)蚀和污损(sun)生(sheng)物的生(sheng)命(ming)活(huo)动(dong)高度(du)依赖特(te)定(ding)的地(di)理位置(zhi)[28]���。实验(yan)室(shi)中(zhong)通(tong)过(guo)培(pei)养污损(sun)生(sheng)物进行研究(jiu)往(wang)往不能(neng)还(hai)原特(te)定海域(yu)的(de)海水理化性质和(he)生(sheng)物多(duo)样性��。因此(ci)有必要在(zai)亚热带湛江(jiang)湾实海(hai)研究污(wu)损生(sheng)物(wu)附(fu)着(zhe)行为(wei)对(dui)钛(tai)合(he)金(jin)的腐(fu)蚀影响(xiang)。
本研(yan)究(jiu)选取(qu)TC4钛合(he)金在湛江(jiang)调顺岛实海中(zhong)挂(gua)样(yang)����,观(guan)察(cha)污损生物(wu)附着(zhe)行为(wei)以(yi)及(ji)试(shi)样(yang)腐(fu)蚀(shi)形貌(mao),结合电(dian)化学测(ce)试和XRD研究(jiu)污损生(sheng)物(wu)附着对(dui)TC4钛(tai)合金的(de)腐蚀(shi)影响(xiang),探索生(sheng)物腐蚀(shi)机理(li),为钛合金在(zai)海洋工程装备(bei)应用提供(gong)一定(ding)的(de)理论和(he)参(can)考(kao)���。
1、实(shi)验材料(liao)和(he)方法(fa)
1.1实验(yan)材(cai)料(liao)
TC4钛合(he)金(jin)化学成(cheng)分如表(biao)1所(suo)示�。
1.2实(shi)验方(fang)法(fa)
1.2.1样品(pin)制备
试样(yang)加(jia)工尺寸为(wei)Φ56mmφ3mm����,用(yong)240#、400#���、800#、1000#砂(sha)纸将试(shi)样(yang)表(biao)面打(da)磨平整(zheng)����,将试样(yang)浸(jin)泡(pao)在丙酮中(zhong)���,放入超(chao)声波(bo)清洗(xi)仪清(qing)洗15分(fen)钟后��,使(shi)用无(wu)水乙(yi)醇(chun)和蒸(zheng)馏水(shui)对(dui)试(shi)样进(jin)行洗(xi)涤���,干(gan)燥封(feng)装����、编(bian)号后放入干(gan)燥(zao)箱(xiang)备(bei)用����。试(shi)样密封(feng)装置(zhi)采(cai)用带(dai)螺纹(wen)管(guan)节,内(nei)部装有金属试样,使(shi)用弹(dan)簧(huang)将导(dao)线压(ya)在金属试样(yang)表面,管节(jie)一(yi)端(duan)使(shi)用环(huan)氧(yang)树(shu)脂(zhi)密封(feng)���,另一(yi)端(duan)采(cai)用(yong)密(mi)封(feng)圈(quan)密封(feng)����。由(you)于密(mi)封(feng)作(zuo)用,试样实(shi)际(ji)上只有一(yi)面暴露于海水(shui)中�。
1.2.2实验(yan)内(nei)容(rong)与(yu)方(fang)法
样品封装后(hou)���,于(yu)2022年9月6日(ri)投(tou)放(fang)在广东湛(zhan)江调(diao)顺岛海(hai)域(yu)�����,浸(jin)入(ru)海(hai)水(shui)中深度(du)为2m,该海(hai)域(yu)全年(nian)月(yue)平(ping)均水(shui)温(wen)16.3℃32.6℃����,盐(yan)度24.2‰26.9‰,pH8.108.22,浊(zhuo)度(du)8.60FTU~13.6FTU��。该海域涨��、落(luo)潮平(ping)均流速分别为0.2m/s和0.24m/s�,涨潮流(liu)速(su)小于(yu)落(luo)潮流速�,其(qi)比值为0.84。其中,大潮(chao)涨、落潮段(duan)平均流速分(fen)别为0.27m/s和(he)0.30m/s;小(xiao)潮(chao)涨�����、落潮段平(ping)均(jun)流速(su)分别(bie)为0.14m/s和(he)0.18m/s�。
本(ben)实验分为(wei)8组����,每(mei)组(zu)设置3个(ge)平(ping)行(xing)样,取(qu)样(yang)周期为5�����、10���、20�、30���、45��、60、75和(he)90d,每个(ge)实验(yan)周期都从(cong)海水(shui)中(zhong)取回(hui)3个(ge)平行(xing)样(yang),首先进(jin)行(xing)电化学(xue)测试�,电解池(chi)采用三电极(ji)体系,参比(bi)电(dian)极(ji)(RE)为饱和KCl甘汞(gong)电极(ji)(SCE),辅(fu)助电极(CE)为20mm×20mm×0.2mm铂电极���,试样(yang)为(wei)工作(zuo)电(dian)极(ji)(WE)�,有效(xiao)面积(ji)24.62cm2,电解质(zhi)为海(hai)水��。在开(kai)路(lu)电位下(xia)对试(shi)样进行(xing)EIS测(ce)试����,频率范围(wei)为0.01~105Hz�����,信号幅(fu)值为(wei)5mV正(zheng)弦波����。极化曲线扫描速(su)率为(wei)0.5mV/s,扫描(miao)区间(jian)±0.01V(相(xiang)对开(kai)路电位(wei)),采(cai)用(yong)Zview软(ruan)件(jian)对EIS进行等效电(dian)路拟(ni)合(he)和(he)分析���。接着使(shi)用(yong)Nikon数码相机(ji)拍摄宏观形(xing)貌(mao),将(jiang)螺(luo)纹(wen)管节拆(chai)开取出(chu)试(shi)样,采用(yong)XRD对(dui)试(shi)样(yang)各(ge)个(ge)浸泡(pao)阶(jie)段腐(fu)蚀产物(wu)进行(xing)分析����,扫描(miao)2角(jiao)度10°~90°,扫(sao)描速率4(°)/min���,步长0.04。
藤壶等大(da)型(xing)污(wu)损(sun)生(sheng)物(wu)使用(yong)镊(nie)子去除钙质(zhi)外壳后��,蒸馏(liu)水(shui)反(fan)复冲洗,然后按照(zhao)GB/T16545-2015《金属和合(he)金的(de)腐蚀(shi)腐蚀试样(yang)上腐蚀(shi)产(chan)物的(de)清除(chu)》配(pei)置除(chu)锈液去(qu)除(chu)藤(teng)壶(hu)基(ji)板和清(qing)洗试(shi)样(yang)表面���,接着(zhe)用无(wu)水乙(yi)醇(chun)洗(xi)涤(di)�,最(zui)后(hou)用蒸馏(liu)水洗涤,干燥(zao)����,最后使(shi)用(yong)徕(lai)卡(ka)超景(jing)深(shen)显微(wei)镜观(guan)察表面微(wei)观(guan)形貌(mao)。
2、结果(guo)与(yu)分(fen)析(xi)
22.1样(yang)品(pin)形貌(mao)观(guan)察(cha)与分析
图(tu)1是TC4钛(tai)合(he)金在(zai)海水中浸泡(pao)不(bu)同(tong)时间的(de)宏(hong)观形貌��。5d,试样(yang)表(biao)面(mian)依然光(guang)亮(liang),有幼(you)体藤壶(hu)附(fu)着(zhe)�。10d,藤壶(hu)基(ji)底(di)直(zhi)径增(zeng)大,“生物(wu)泥”肉眼(yan)可见(jian)�。20d�,藤(teng)壶(hu)数(shu)量与(yu)个(ge)体呈上(shang)升趋(qu)势(shi),个别(bie)藤(teng)壶(hu)基底(di)直径达(da)7mm,试(shi)样约50%面(mian)积被(bei)覆盖�����,“生物(wu)泥”厚(hou)度明(ming)显增大�,水螅(xi)���、管栖(qi)多毛类泥(ni)管和石(shi)灰(hui)质(zhi)管等开(kai)始附着,这(zhe)些均(jun)为我国南(nan)海(hai)污(wu)损生(sheng)物(wu)季节性(xing)优势(shi)物种(zhong)[29],也是(shi)“生物(wu)泥”主(zhu)要构成,如图(tu)2所示���。“生物(wu)泥(ni)”的形(xing)成是(shi)细(xi)菌和(he)硅藻等微(wei)生(sheng)物膜加上有机或(huo)无(wu)机(ji)颗(ke)粒(li)的(de)运动、黏(nian)附和(he)沉(chen)积作(zuo)用(yong)�,使微生(sheng)物(wu)膜(mo)增厚形成(cheng)“生(sheng)物(wu)泥(ni)”[22]��。30~45d,未观察到其他新(xin)物种附(fu)着,藤(teng)壶基(ji)底(di)直(zhi)径(jing)增大���、数(shu)量增(zeng)加(jia)�����,覆(fu)盖(gai)面(mian)积(ji)达(da)90%以上(shang),表明(ming)钛(tai)合(he)金具(ju)有(you)较(jiao)好(hao)的(de)生(sheng)物相容(rong)性(xing),是海洋生(sheng)物的理(li)想栖息地(di)[30]���。同(tong)时(shi)��,部(bu)分藤壶死(si)亡(wang)形成空壳���,藤(teng)壶(hu)空(kong)壳(ke)在海水(shui)冲(chong)刷下(xia)脱落,但(dan)藤壶(hu)基底(di)并未脱(tuo)离试样��,表(biao)明(ming)藤(teng)壶(hu)胶(jiao)具有(you)较(jiao)强(qiang)粘性[31−32]。
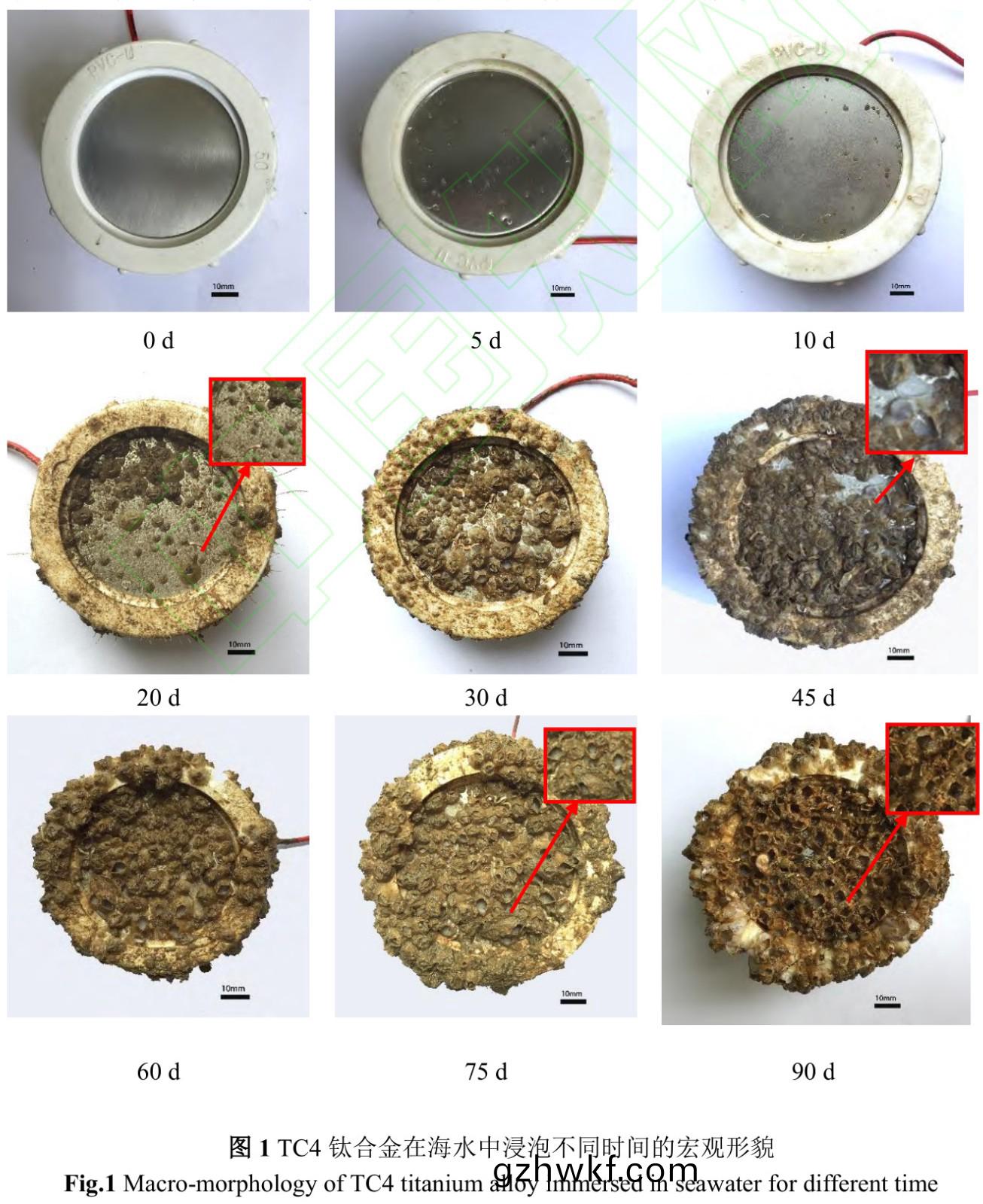

60~75d,此(ci)时藤(teng)壶依然(ran)是(shi)试(shi)样表(biao)面污(wu)损生(sheng)物的(de)优势(shi)物种����,此(ci)时污(wu)损(sun)生(sheng)物(wu)覆盖面积比高达99%。温差(cha)过大可(ke)造(zao)成(cheng)藤(teng)壶大面积死亡(wang)[33−36],而(er)本(ben)实验(yan)是(shi)在(zai)秋(qiu)冬(dong)季(ji)进(jin)行�,75~90d时湛(zhan)江(jiang)海(hai)域(yu)进入冬(dong)季(ji)���,受季(ji)节影(ying)响温(wen)度骤(zhou)变��,导致藤壶(hu)大部分(fen)死亡�����,死(si)亡率(lv)达95%。
图(tu)3是TC4钛合(he)金在(zai)海(hai)水(shui)中浸泡不同(tong)时间(jian)藤(teng)壶(hu)附(fu)着数(shu)量(liang)及面(mian)积占(zhan)比(bi)。从(cong)图(tu)中可(ke)以(yi)看出10d时藤壶附着(zhe)数量最少,为(wei)1.3pcs/cm2�����,结合图1可(ke)知在(zai)试验(yan)初期(qi)����,试样(yang)表(biao)面(mian)较为(wei)光(guang)滑(hua),幼体藤壶附着(zhe)强度较小(xiao)[33]��,部(bu)分(fen)幼(you)体藤壶(hu)在(zai)海(hai)水(shui)冲刷(shua)下脱(tuo)落(luo)或死(si)亡脱落�����。20d时(shi)�,试(shi)样表(biao)面(mian)藤(teng)壶(hu)数量明显(xian)增(zeng)多(duo),个体基(ji)底(di)直径明显(xian)增(zeng)大。随着试验时(shi)间延(yan)长,试样表(biao)面粗糙度和(he)“生物泥(ni)”厚度逐渐(jian)增(zeng)大(da),且部分藤(teng)壶生长成(cheng)熟(shu),开(kai)始(shi)繁殖(zhi)[37],使得试(shi)样(yang)表面藤(teng)壶附着(zhe)数量在(zai)30d时(shi)最多(duo),为4.5pcs/cm2����。之(zhi)后(hou)藤壶基(ji)底(di)直(zhi)径不(bu)断(duan)增(zeng)大��,附(fu)着空间被压缩,幼(you)体(ti)藤(teng)壶挤压(ya)脱(tuo)落(luo)以及(ji)部(bu)分藤壶死亡脱(tuo)落����,藤(teng)壶(hu)附(fu)着(zhe)数量(liang)波动(dong)变化�����,但整(zheng)体(ti)覆盖(gai)面(mian)积占(zhan)比(bi)几乎(hu)不(bu)变。
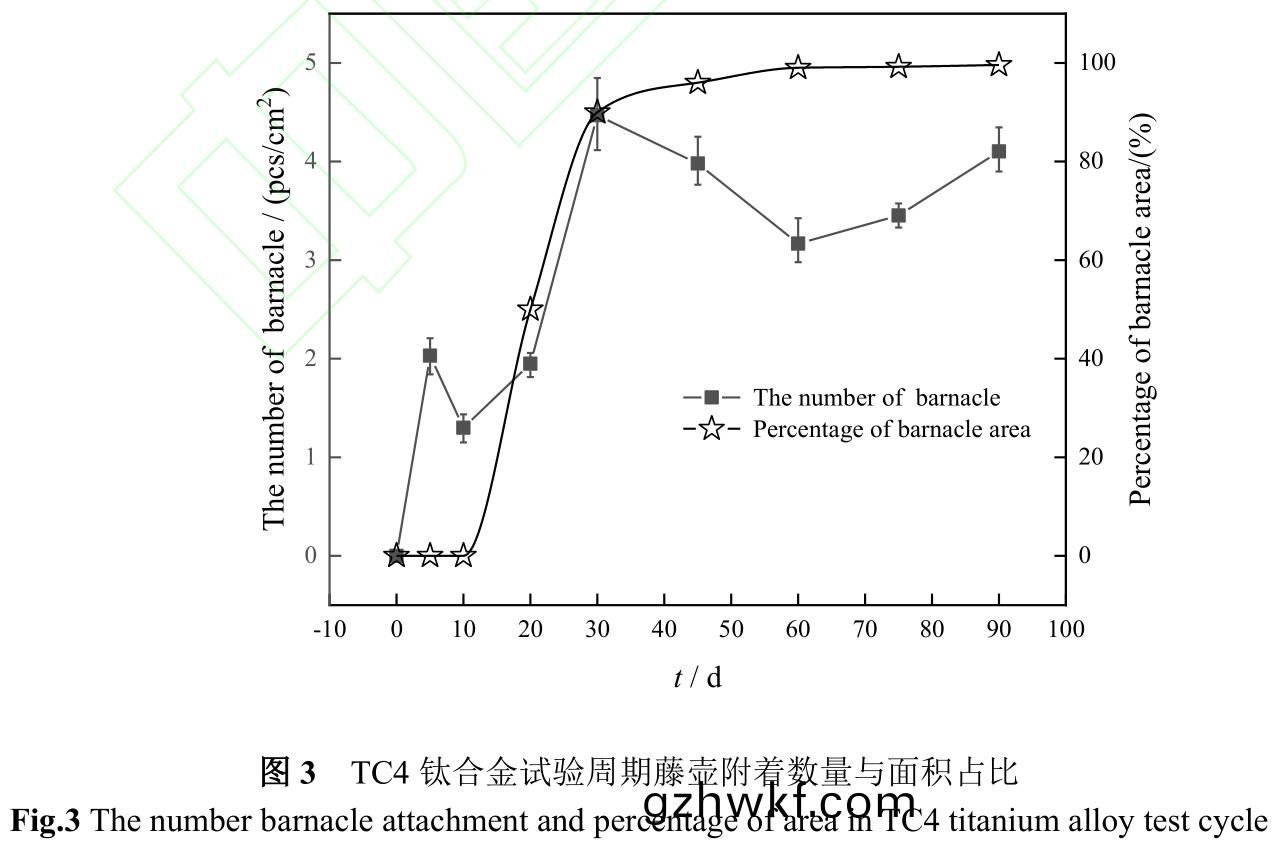
图4是(shi)TC4钛合金(jin)去除表面附着(zhe)物后(hou)的微观(guan)形(xing)貌图。结合(he)图1和图(tu)4可知(zhi)���,在海(hai)水中(zhong)浸泡的(de)前(qian)10d,钛(tai)合(he)金(jin)表(biao)面主(zhu)要是(shi)由微生物组(zu)成的生(sheng)物(wu)膜,同(tong)时也是(shi)钛合金钝(dun)化(hua)膜(mo)形成阶(jie)段(duan),微生物(wu)膜(mo)的(de)形成造(zao)成(cheng)介(jie)质(zhi)不均(jun)匀(yun)性导(dao)致(zhi)钛合金(jin)钝(dun)化(hua)膜不均匀����,表面(mian)呈现密集(ji)的小而浅的缺陷;在(zai)海(hai)水中(zhong)浸(jin)泡30d后(hou)���,钛合(he)金表面(mian)主(zhu)要(yao)污(wu)损(sun)生(sheng)物演(yan)替为(wei)藤(teng)壶,随(sui)藤(teng)壶生长与死(si)亡�,在(zai)生(sheng)长过程中(zhong)藤(teng)壶(hu)体(ti)现较好(hao)的阻(zu)隔作(zuo)用,钛(tai)合(he)金(jin)钝(dun)化膜(mo)基(ji)本(ben)完(wan)整,表(biao)面(mian)呈(cheng)现小(xiao)而浅的缺(que)陷;藤壶(hu)死亡(wang)后(hou)���,藤(teng)壶阻隔(ge)性(xing)下(xia)降(jiang)�����,藤(teng)壶(hu)内部有机(ji)质(zhi)分(fen)解造(zao)成(cheng)低(di)氧(yang)海(hai)水环境(jing)����,从而(er)使(shi)钛合(he)金钝化(hua)膜再生(sheng)困难(nan),并且藤(teng)壶(hu)下(xia)钛(tai)合金(jin)与藤(teng)壶外钛(tai)合(he)金(jin)之(zhi)间(jian)形(xing)成强(qiang)烈(lie)的(de)氧(yang)浓差(cha),构(gou)成(cheng)氧(yang)浓差电池(chi),藤壶下(xia)钛(tai)合金(jin)发生(sheng)较快(kuai)腐(fu)蚀(shi),呈现(xian)大而(er)深的(de)腐蚀坑。
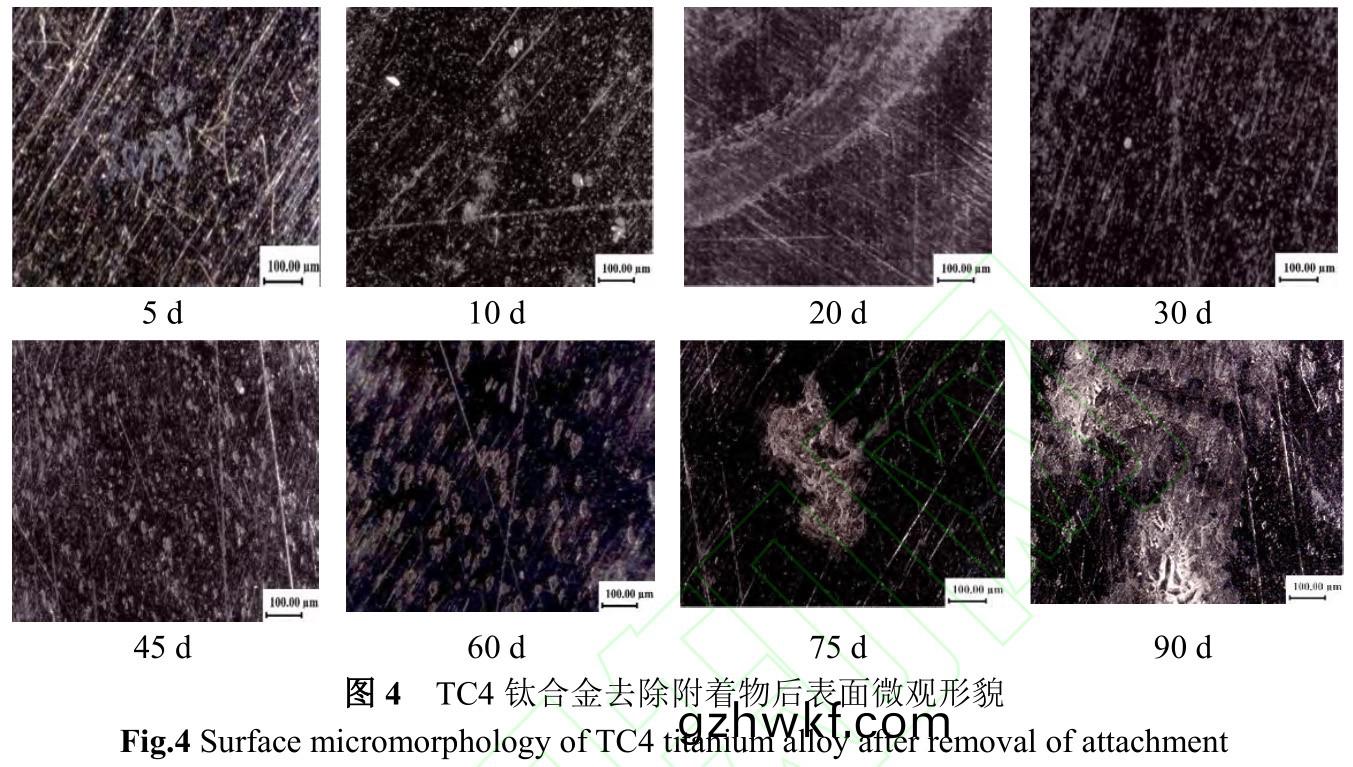
图5是(shi)试(shi)样表面3D微观图���。试(shi)验中后期(45~90d)��,试(shi)样(yang)表(biao)面无(wu)藤壶附(fu)着位(wei)置(zhi)或(huo)藤(teng)壶与(yu)藤壶(hu)空(kong)隙之间(jian)无明(ming)显蚀坑(keng),而藤壶(hu)附着(zhe)位置(zhi)出(chu)现(xian)蚀(shi)坑(keng)�����。随(sui)着(zhe)时间(jian)延(yan)长(zhang)�����,藤(teng)壶附(fu)着位置(zhi)点蚀(shi)更加密(mi)集或形(xing)成溃(kui)疡(yang)坑(keng),60d时(shi)溃疡(yang)坑直径高(gao)达1.5mm���,90d时蚀(shi)坑最深(shen)可达0.14mm���。
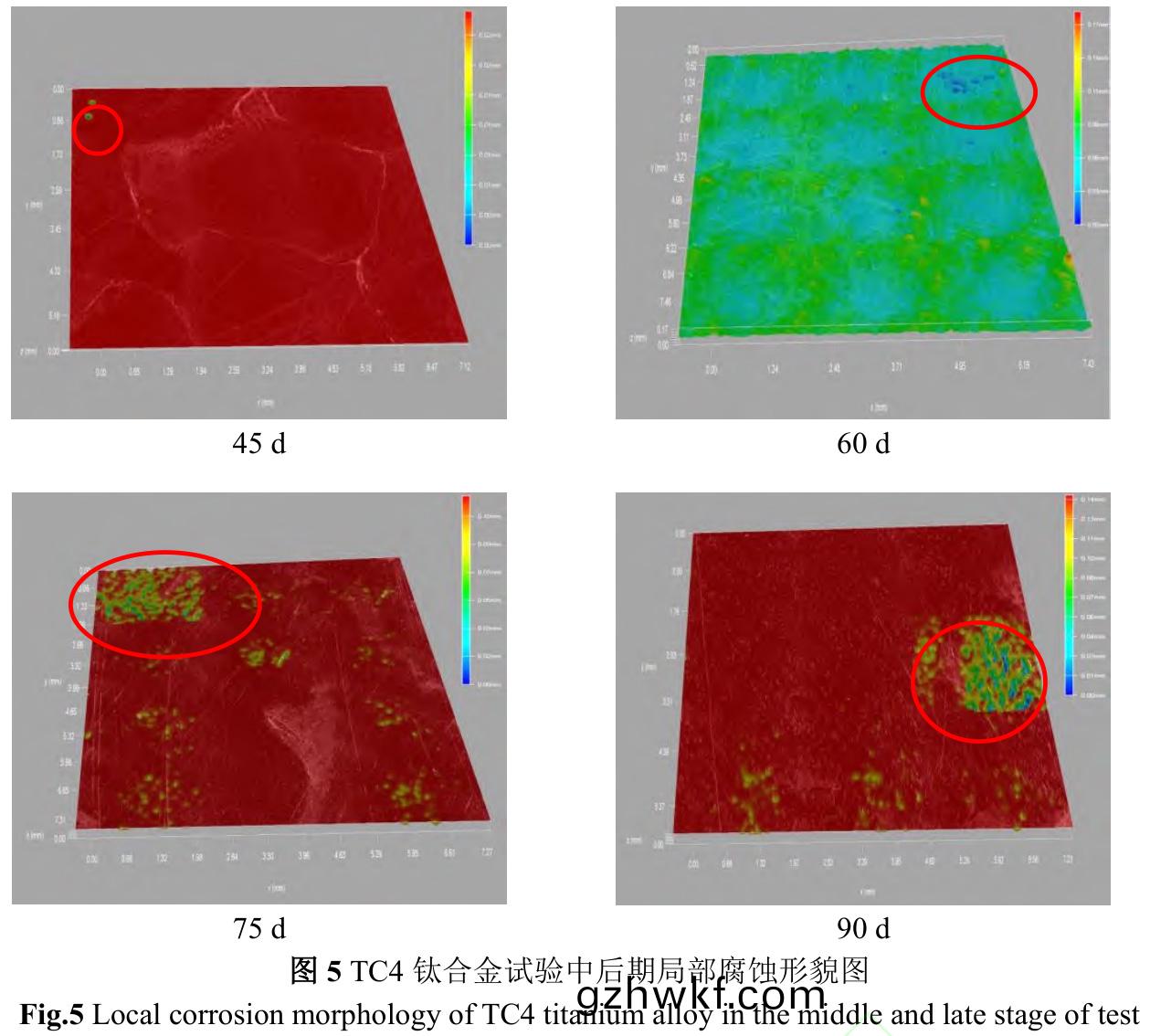
藤壶(hu)引起(qi)腐蚀(shi)需(xu)要具备(bei)三(san)个(ge)条件(jian):附着(zhe)藤(teng)壶死亡�、微生(sheng)物(wu)对(dui)死亡藤(teng)壶(hu)有机体作(zuo)用和(he)构(gou)成宏(hong)观电池(chi)[38]。海(hai)洋(yang)污(wu)损生物(wu)附(fu)着(zhe)以及(ji)生(sheng)命(ming)活动(dong)新陈代(dai)谢(xie)产生大量代谢产(chan)物和酸(suan)性物(wu)质,富集在试(shi)样表(biao)面形(xing)成(cheng)生物(wu)膜,从而在(zai)金属(shu)和(he)生物膜之间形(xing)成局部(bu)厌氧区,进(jin)而(er)破(po)坏钝(dun)化膜���,为海(hai)水中(zhong)腐(fu)蚀性离子(zi)提供(gong)通道�����,促进(jin)腐蚀(shi)[39]。由图1和图5可(ke)知,藤壶在(zai)30d开始(shi)出(chu)现死(si)亡,钙质外壳被(bei)破坏(huai)和(he)有机(ji)体被海水中(zhong)微(wei)生物分(fen)解(jie)�,为Cl−与材料表(biao)面(mian)的(de)接(jie)触(chu)�����、传输提供(gong)路径(jing),从而导(dao)致(zhi)钝(dun)化膜破(po)裂(lie)�����。同(tong)时(shi)藤壶壳(ke)内(nei)第一触角位(wei)置易形(xing)成活化点成为(wei)阳(yang)极(ji)与壳外(wai)金属(shu)作(zuo)为(wei)阴极(ji)构成(cheng)宏(hong)观电池(chi)��,促进(jin)局部(bu)腐(fu)蚀。不同(tong)生(sheng)长阶段(duan)的藤壶(hu)对(dui)TC4钛合金的腐蚀机制(zhi)有所不(bu)同(tong),结构(gou)完(wan)整的活(huo)体藤壶(hu)钙(gai)质外壳和致(zhi)密(mi)藤(teng)壶(hu)胶(jiao)能(neng)够(gou)有效隔离(li)外(wai)界腐蚀(shi)介质���,死(si)亡(wang)藤壶(hu)则因壳体结(jie)构(gou)不(bu)完整与(yu)有机(ji)体(ti)被(bei)微(wei)生物(wu)分(fen)解(jie)�,反(fan)而促(cu)进腐(fu)蚀(shi)���。
2.2电(dian)化学(xue)测(ce)试结果与(yu)分(fen)析(xi)
(1)极(ji)化(hua)曲线
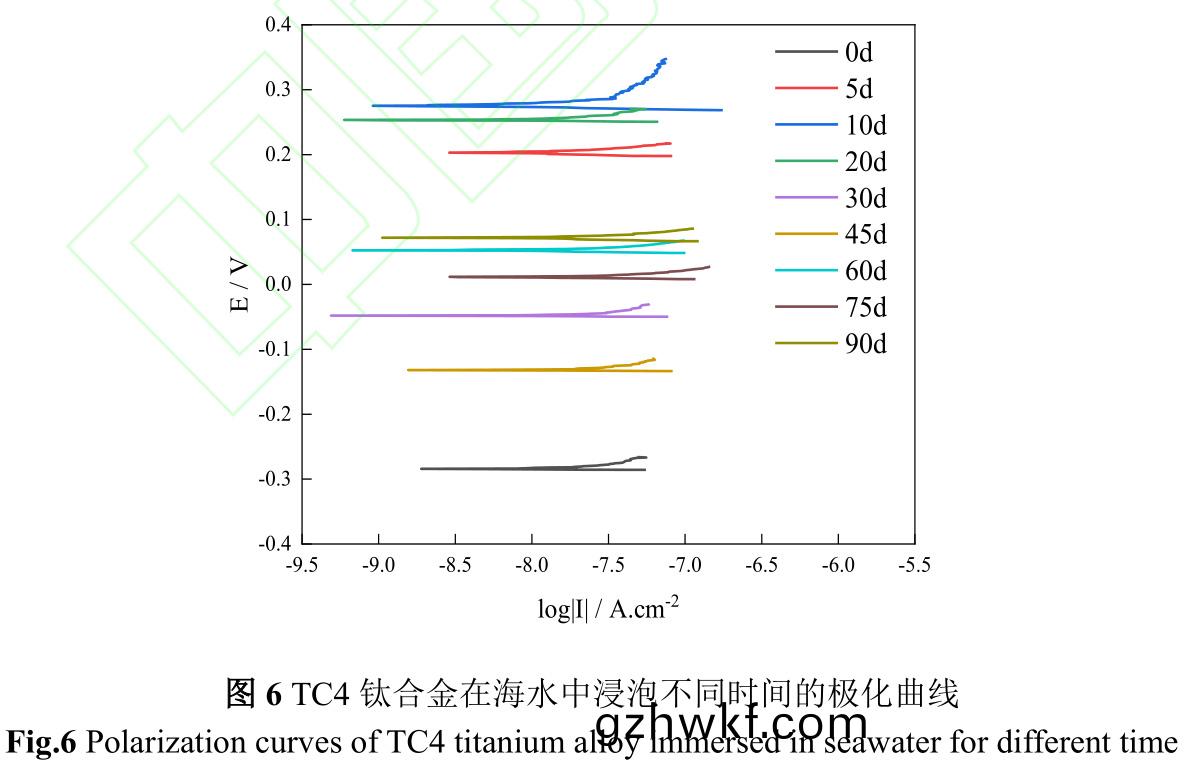
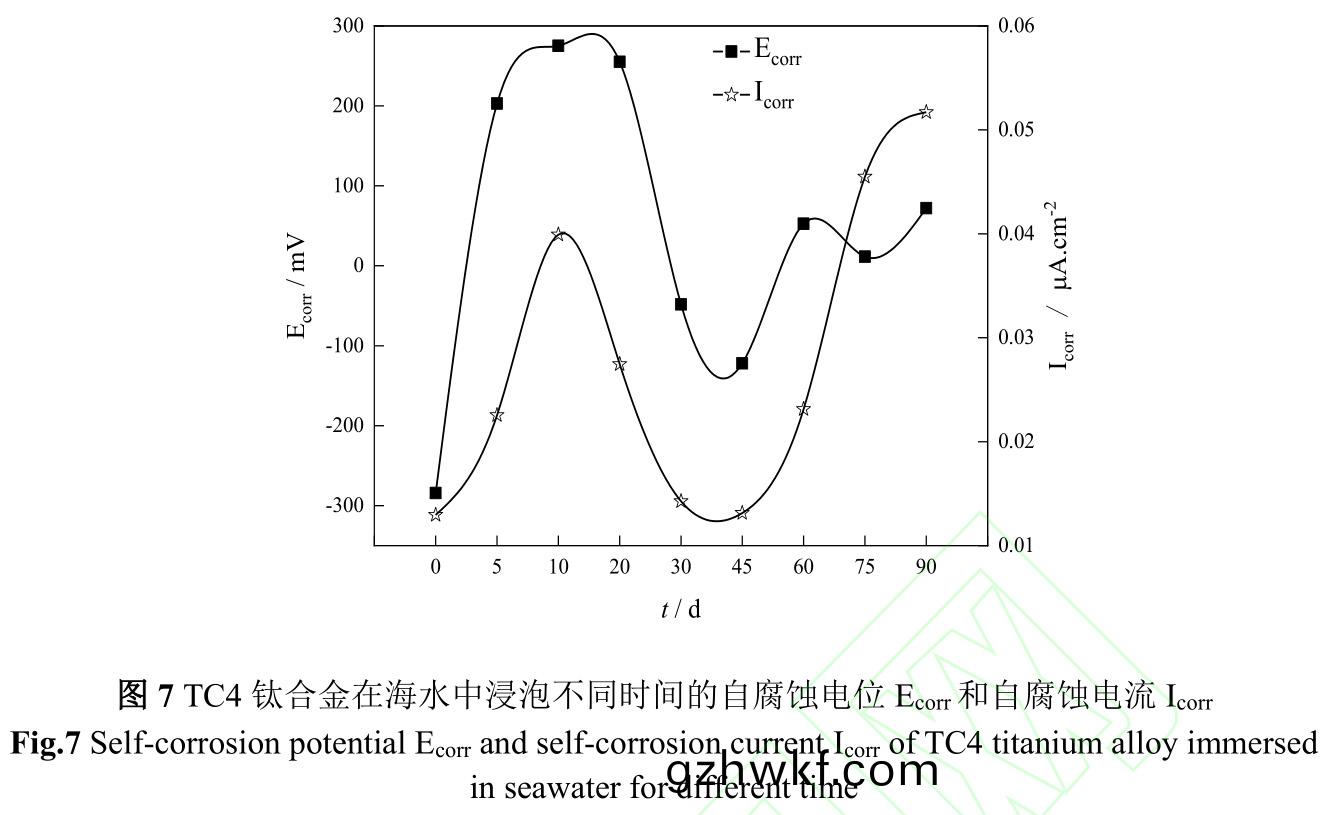
图6是(shi)TC4钛合(he)金(jin)在海(hai)水(shui)中浸泡不同(tong)时(shi)间极(ji)化(hua)曲线,图7是TC4钛合金在海(hai)水(shui)中(zhong)浸泡(pao)不(bu)同时间(jian)的自腐蚀(shi)电(dian)位(wei)(Ecorr)和自(zi)腐(fu)蚀电(dian)流(Icorr)����。由(you)图(tu)6、7可(ke)知,TC4钛(tai)合(he)金在海(hai)水(shui)中初始腐蚀(shi)电(dian)位(wei)较(jiao)负(fu),为−284.2mV����,随(sui)着试(shi)验时间延(yan)续,腐(fu)蚀(shi)电位(wei)急(ji)剧(ju)正(zheng)移,腐(fu)蚀(shi)电(dian)流伴(ban)随腐蚀(shi)电位(wei)而升(sheng)高(gao)�,在(zai)第10d�����,腐(fu)蚀(shi)电(dian)流达到最(zui)大,该(gai)阶(jie)段金(jin)属表(biao)面(mian)阳(yang)极(ji)溶解过(guo)程加速���,从而增加了钛(tai)合(he)金(jin)腐蚀(shi)速度(du)��。第10d至(zhi)45d,钛合金腐(fu)蚀电位(wei)逐渐(jian)下降,腐蚀(shi)电流(liu)亦逐(zhu)渐(jian)下(xia)降(jiang),结(jie)合图(tu)1污损生物(wu)由(you)微(wei)生物(wu)、小(xiao)型生(sheng)物(wu)组成(cheng)生(sheng)物淤(yu)泥层向以藤(teng)壶(hu)为(wei)主(zhu)要(yao)的大型(xing)污损生物(wu)层转(zhuan)变,金(jin)属表(biao)面阳(yang)极溶(rong)解(jie)过(guo)程(cheng)受到抑制(zhi),从(cong)而(er)减(jian)缓钛合(he)金(jin)腐蚀(shi)速度(du)��。第45d后��,以(yi)藤(teng)壶(hu)为主的大(da)型(xing)污损(sun)生(sheng)物(wu)大(da)面积死亡(wang),改(gai)变了(le)钛合金表面(mian)腐蚀环境�����,导(dao)致(zhi)腐蚀电(dian)位(wei)升(sheng)高(gao)的同(tong)时腐蚀电流(liu)也升(sheng)高(gao)�����。
(2)EIS
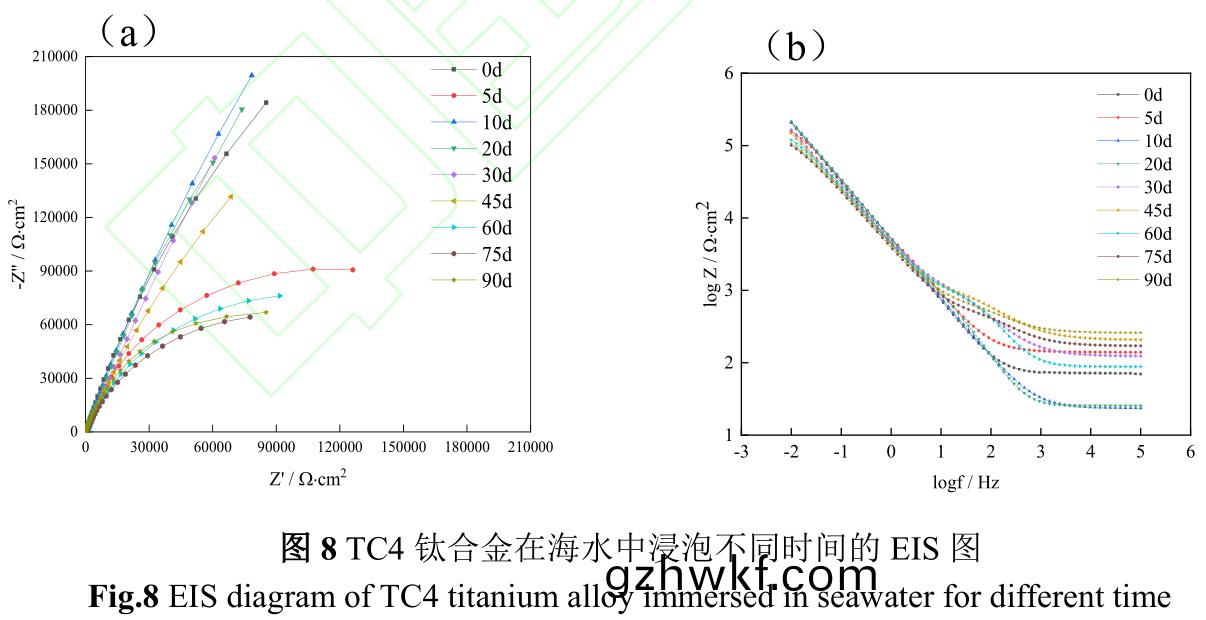
图(tu)8为TC4钛合(he)金在(zai)海(hai)水(shui)中(zhong)浸(jin)泡不(bu)同(tong)时(shi)间(jian)的EIS图(tu)����。由图1�、2、7和图(tu)8中的Nyquist图可(ke)知(zhi),TC4钛(tai)合(he)金在浸(jin)泡(pao)初(chu)期(0~10d)��,生(sheng)物膜(mo)附(fu)着(zhe)与(yu)金(jin)属(shu)表面钝(dun)化(hua)膜(mo)致密化同(tong)时(shi)发生,容(rong)抗(kang)弧半(ban)径先减小(xiao)再(zai)增大(da)�,第10d时达到最大,此时材(cai)料(liao)耐蚀性较(jiao)好�。试验(yan)中(zhong)期(20~45d)�,容(rong)抗(kang)弧(hu)半径逐渐减小,但(dan)幅度(du)相(xiang)对较(jiao)小,生物膜的(de)形成(cheng)-脱落(luo)以(yi)及(ji)藤(teng)壶(hu)为主(zhu)大型(xing)污损生(sheng)物(wu)的(de)附着-生长(zhang),影(ying)响(xiang)了钝(dun)化(hua)膜再生(sheng)�。试验(yan)后(hou)期(qi)(60~90d)�,容(rong)抗弧半径(jing)波动变化(hua)但(dan)明(ming)显小于(yu)试验(yan)前(qian)中期(0~45d),表(biao)明试(shi)验(yan)后(hou)期(qi)材料耐蚀(shi)性(xing)较(jiao)差(cha),腐蚀趋(qu)势较(jiao)大�。
为(wei)定(ding)量(liang)分析TC4钛合金(jin)与溶液(ye)界(jie)面(mian)之(zhi)间(jian)的变化(hua)关(guan)系(xi),采用图9的(de)等效(xiao)电(dian)路(lu)图(tu)对EIS进行(xing)拟(ni)合�����。试样(yang)浸(jin)泡(pao)前(qian)�,表面没有(you)生(sheng)物膜(mo)和污(wu)损(sun)生(sheng)物附着(zhe)����,采(cai)用(yong)单(dan)层(ceng)膜(mo)模(mo)型(xing)Rs(CPE1Rct)���,试(shi)样(yang)浸泡到(dao)海(hai)中,生(sheng)物膜(mo)和污(wu)损生(sheng)物(wu)的影(ying)响采(cai)用(yong)双层(ceng)膜(mo)模型(xing)Rs(CPE1(Rf(CPE2Rct)))����。其中,Rs是溶(rong)液电(dian)阻,CPE1是膜(mo)电容(rong)�,Rf是膜(mo)电阻(zu)����,Rct是(shi)电(dian)荷转移(yi)电阻(zu),CPE2是溶液(ye)双(shuang)电层(ceng)电(dian)容(rong)���。以Rs(CPE1Rct)和(he)Rs(CPE1(Rf(CPE2Rct)))两(liang)种物(wu)理模型(xing)拟合的等(deng)效电路参数如表2所示����。
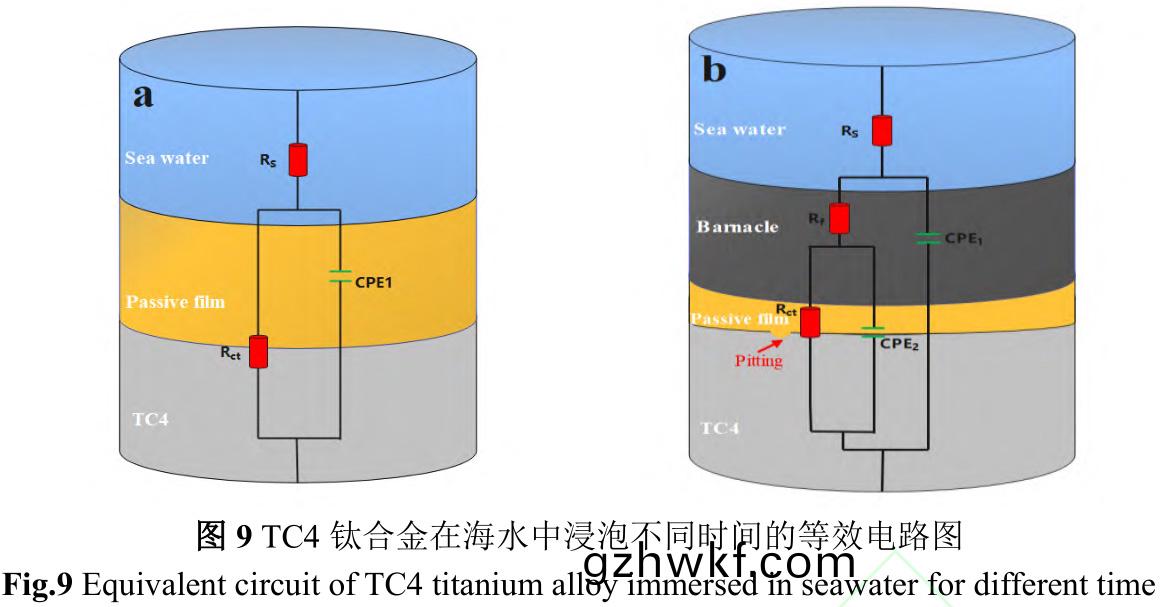
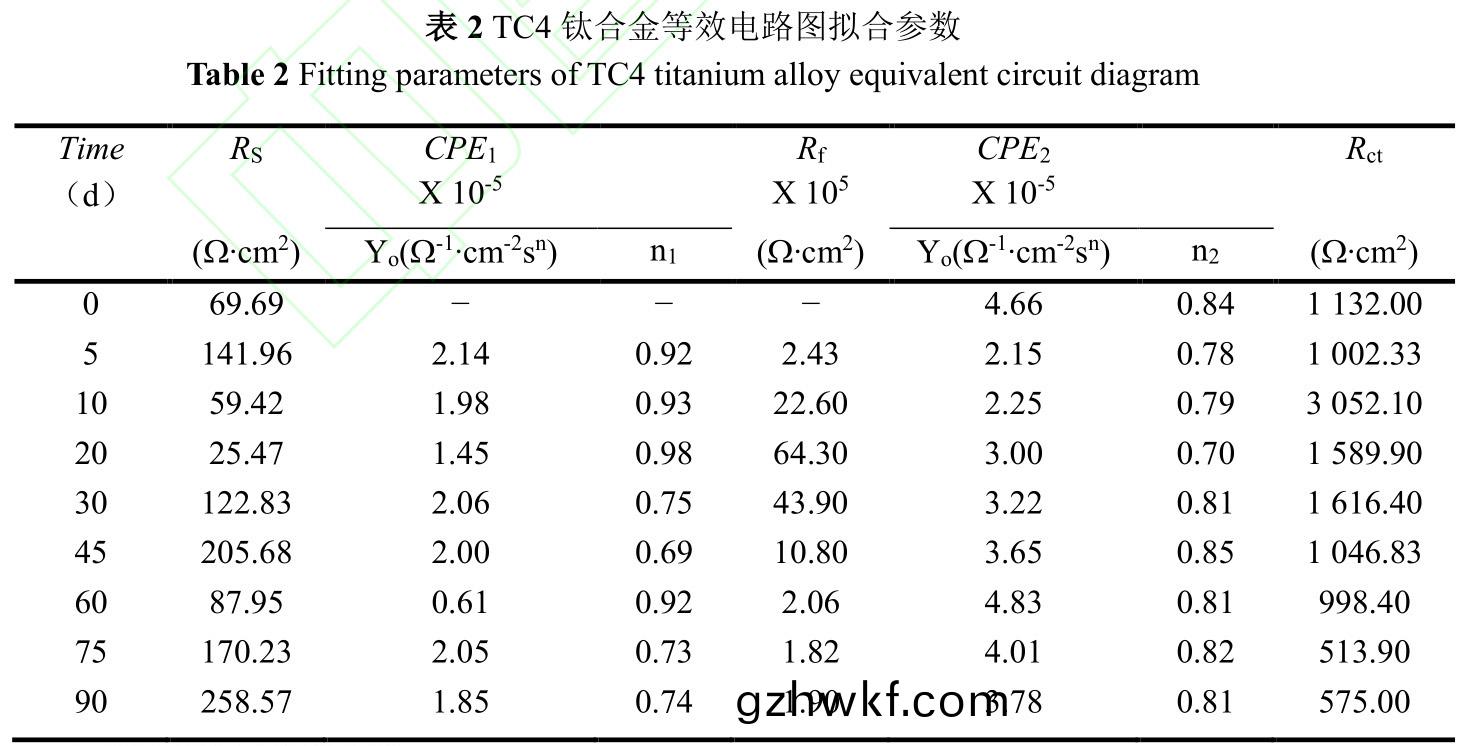
电(dian)化学(xue)测试溶液(ye)取自海(hai)中(zhong),不(bu)同(tong)试(shi)验周期海水浊度����、盐度等(deng)理化(hua)参(can)数(shu)受(shou)气(qi)候影响(xiang)并不一(yi)致,导致溶(rong)液体(ti)系(xi)相对(dui)不(bu)稳(wen)定,其(qi)中(zhong)溶液(ye)电阻(zu)Rs最(zui)大(da)值(zhi)为258.57Ω.cm2���,最(zui)小(xiao)值为25.47Ω.cm2�,最(zui)大偏差为233.1Ω.cm2。Rp为极(ji)化(hua)电(dian)阻(zu)��,等(deng)于(yu)电荷转(zhuan)移(yi)电阻Rct与(yu)膜(mo)电阻Rf的代(dai)数和(he),用于(yu)衡(heng)量材料(liao)抵(di)抗腐(fu)蚀介(jie)质(zhi)穿透(tou)能力(li)的大小�����,与金属(shu)腐蚀速(su)度呈负(fu)相关。从(cong)拟合结(jie)果(guo)来(lai)看(kan),在试(shi)验(yan)初(chu)期(0~10d),第5d的(de)Rp明(ming)显小于(yu)第(di)10d�,表明浸(jin)泡初期钝化(hua)膜(mo)致密(mi)性(xing)较(jiao)差��,该阶段(duan)污(wu)损生(sheng)物(wu)膜(mo)附(fu)着与钛合(he)金(jin)表面钝化膜(mo)增(zeng)厚��、致(zhi)密化(hua)过(guo)程(cheng)。第10d至(zhi)45d的(de)Rp依(yi)旧(jiu)处于较高(gao)水(shui)平���,结(jie)合图1可知(zhi)第10d后(hou)污(wu)损生(sheng)物(wu)由微(wei)生(sheng)物(wu)�、小型(xing)生物(wu)组(zu)成(cheng)生(sheng)物淤泥层(ceng)向(xiang)以藤壶为主(zhu)要污(wu)损(sun)生物的(de)大(da)型(xing)污损生(sheng)物(wu)层(ceng)转(zhuan)变��,大(da)型(xing)污(wu)损(sun)生(sheng)物层(ceng)相对(dui)生(sheng)物淤(yu)泥(ni)层(ceng)输(shu)氧效率(lv)高��,钝(dun)化膜(mo)再生(sheng)能力(li)较(jiao)强��,阻滞(zhi)了阳(yang)极(ji)溶(rong)解�,腐(fu)蚀(shi)速度(du)有(you)所降低(di)。试验后(hou)期(60~90d)����,藤(teng)壶大(da)量(liang)死亡(wang)���,死亡藤(teng)壶造(zao)成(cheng)钛合(he)金(jin)表(biao)面介(jie)质性(xing)质不(bu)均匀化(hua)[40],影响(xiang)钛合金(jin)表面(mian)钝化(hua)膜形(xing)成(cheng)与(yu)再(zai)生��,使(shi)Rp降低(di),从而导(dao)致腐蚀(shi)速(su)度(du)增(zeng)大(da)�。整个(ge)试验(yan)中,污损生物(wu)附(fu)着影响钛合金表(biao)面钝化(hua)膜的形成与(yu)再(zai)生(sheng)��,从而(er)影(ying)响整(zheng)个腐(fu)蚀(shi)进(jin)程。
2.3腐(fu)蚀(shi)产(chan)物及(ji)机理分析(xi)
图10是TC4钛(tai)合金(jin)在海水(shui)中(zhong)浸泡(pao)不(bu)同时(shi)间(jian)的(de)XRD图(tu)。TC4钛合(he)金(jin)为钛铝(lv)合金(jin)����,在(zai)海(hai)水(shui)环境(jing)中,TC4钛(tai)合金表(biao)面形成含(han)有(you)TiO2和Al2O3的(de)钝化膜,而(er)CaCO3主要(yao)来源于(yu)钙质外(wai)壳(ke)污(wu)损生(sheng)物�。CaCO3衍(yan)射(she)峰从第10d出现,到第20d峰(feng)型尖(jian)锐(rui)�����,此(ci)演变(bian)规律(lv)与污(wu)损生(sheng)物(wu)演替规(gui)律相同(tong)���。在20~45d期(qi)间(jian)�����,CaCO3衍(yan)射峰呈增(zeng)强(qiang)-减(jian)弱(ruo)-增(zeng)强的波(bo)动变化�����,这(zhe)是因(yin)为钙(gai)质(zhi)外壳(ke)污(wu)损生(sheng)物(wu)局(ju)部附着(zhe),而XRD分(fen)析区域与钙(gai)质外(wai)壳(ke)污损(sun)生物附着(zhe)区域(yu)错(cuo)致(zhi)所致(zhi)。
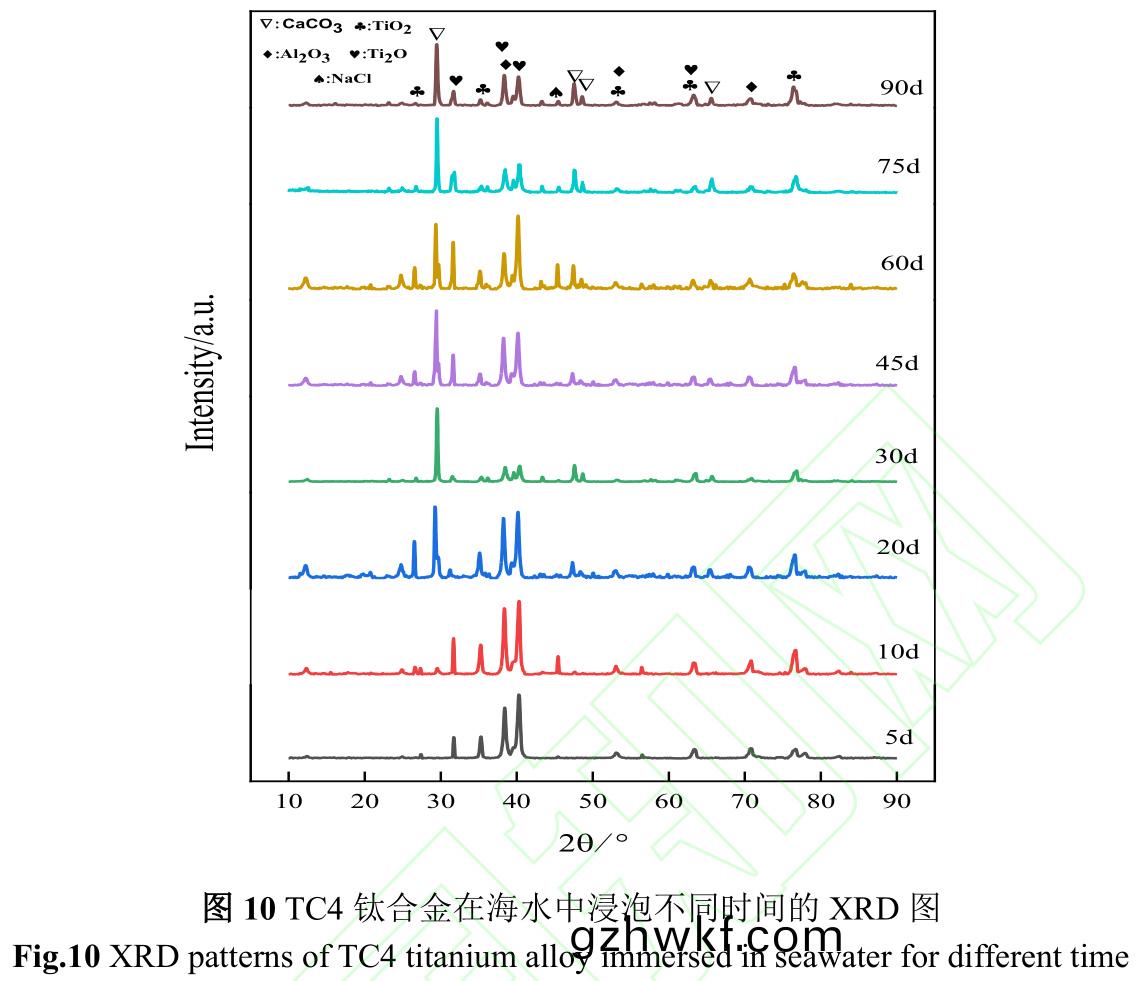
图11是(shi)TC4钛合(he)金在海(hai)水中的(de)腐蚀机(ji)理(li)图(tu)�����。钛合金具(ju)有优良(liang)的耐蚀性(xing)是(shi)因为表(biao)面易(yi)形(xing)成(cheng)钝(dun)化膜,钛(tai)合(he)金在海水(shui)中(zhong)钝(dun)化(hua)反应如(ru)下(xia):
Ti+2H2O→TiO2+4H++4e−(1)
2TiO2+2H++2e−→Ti2O3+H2O(2)
Ti2O3稳定性(xing)较(jiao)差(cha)�,会再次(ci)被(bei)氧化成(cheng)TiO2:
Ti2O3+H2O→2TiO2+2H++2e−(3)
钛合金钝(dun)化和污损(sun)生(sheng)物(wu)附(fu)着(zhe)几(ji)乎(hu)同(tong)时发生。Venkatesan等[41]研(yan)究(jiu)表(biao)明(ming)海(hai)洋(yang)污(wu)损生(sheng)物附(fu)着(zhe)是(shi)长(zhang)期(qi)且复(fu)杂(za)的自(zi)然选择(ze)��、生态(tai)演(yan)替过程(cheng)�����。通常(chang)材(cai)料(liao)浸(jin)没在海水后�����,几分钟(zhong)内被多聚(ju)糖(tang)和(he)糖蛋(dan)白(bai)等(deng)有机(ji)分子与(yu)无机化(hua)合物(wu)快(kuai)速(su)黏(nian)附(fu),形(xing)成基(ji)膜(mo)�。浮游(you)细(xi)菌利用范德(de)华(hua)力(li)等弱(ruo)作(zuo)用力在(zai)基膜上附着���,通(tong)过新陈代(dai)谢产(chan)生(sheng)具有黏(nian)性的细胞(bao)外高(gao)聚物(wu)[42]���,使(shi)附(fu)着逐(zhu)渐(jian)牢固,最终形成(cheng)生(sheng)物(wu)膜(mo)。生(sheng)物(wu)膜形成为藤壶幼虫等大(da)型污(wu)损生物(wu)附着提(ti)供了(le)良(liang)好条(tiao)件���。生(sheng)物膜形(xing)成和(he)大型(xing)污(wu)损(sun)生(sheng)物(wu)附着使(shi)试样(yang)表面(mian)形(xing)成“生物封(feng)闭滞留层(ceng)”��,试(shi)样(yang)不(bu)再直(zhi)接与(yu)海(hai)水接触。“生物封闭(bi)滞留(liu)层”是封(feng)闭或半封(feng)闭(bi)状(zhuang)态(tai),污(wu)损生物在(zai)试(shi)样表(biao)面(mian)所占(zhan)面(mian)积(ji)越大(da),对材(cai)料(liao)整体性保(bao)护(hu)越(yue)好(hao)[43]。
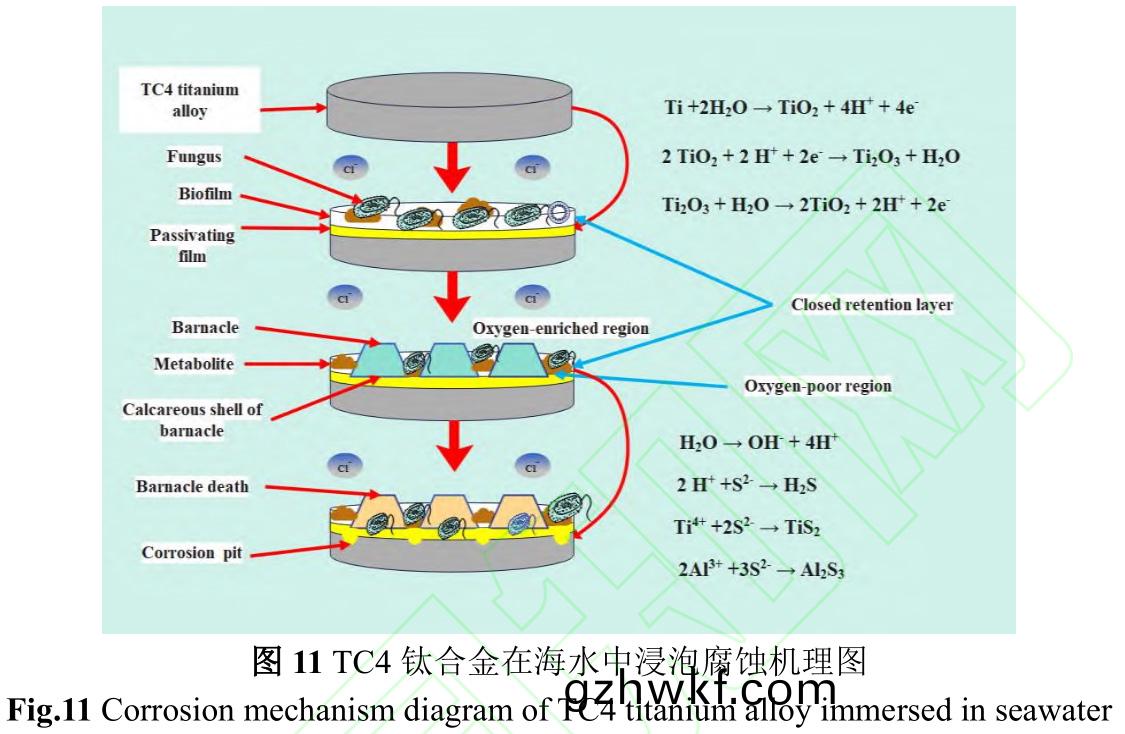
但(dan)由(you)于(yu)大型污(wu)损生物(wu)不均匀附(fu)着以及存(cun)在(zai)耗氧(yang)和(he)产(chan)氧(yang)生物����,易造成不(bu)同(tong)区(qu)域(yu)氧(yang)浓度(du)差异(yi),形(xing)成(cheng)氧浓(nong)差(cha)电(dian)池����,富氧区(qu)作为(wei)阴极(ji)接受(shou)电子发生还原反(fan)应(ying)(反(fan)应4),贫氧区(qu)作为阳(yang)极(ji)失(shi)去(qu)电(dian)子(zi)发(fa)生氧(yang)化(hua)反应(反(fan)应5)����,由(you)表1可(ke)知TC4钛合金(jin)主(zhu)要成(cheng)分为Ti和(he)Al��,那么(me)阳(yang)极发(fa)生是主要(yao)反(fan)应(ying)如反(fan)应6、7所(suo)示,形(xing)成局(ju)部(bu)腐(fu)蚀(反(fan)应8���、9)��,表(biao)现(xian)为如图(tu)5所示(shi)的溃(kui)疡(yang)坑(keng)或(huo)较(jiao)为(wei)密(mi)集(ji)点蚀(shi)��。
O2+2H2O+4e−→4OH−(4)
M→M+++2e−(5)
Ti−2e−→Ti2+(6)
Al−3e−→Al3+(7)
Al3++3Cl−→AlCl3(8)
4AlCl3+3O2→2Al2O3+6Cl2(9)
Sangeetha等(deng)[44]认(ren)为藤(teng)壶(hu)胶(jiao)能够破坏(huai)钝(dun)化膜(mo)����,为海水(shui)中(zhong)的(de)Cl−等(deng)卤素(su)腐(fu)蚀(shi)性离子提(ti)供了通(tong)道�,从(cong)而(er)导(dao)致(zhi)腐蚀不断(duan)发展���。而(er)Eashwar等(deng)[45]认(ren)为藤壶死(si)亡(wang)是材料发(fa)生(sheng)局(ju)部(bu)腐蚀(shi)的(de)先(xian)决条(tiao)件(jian)。图(tu)1中(zhong)第(di)30d开始发(fa)现有藤(teng)壶死亡����,结(jie)合图5的3D微(wei)观图(tu)局部腐(fu)蚀(shi)情况(kuang)可知与(yu)Eashwar等(deng)的研(yan)究更(geng)相符。藤(teng)壶死(si)亡后(hou),藤(teng)壶有(you)机体(ti)被好氧细(xi)菌分解产(chan)生(sheng)酸性(xing)物质�����,降(jiang)低(di)藤(teng)壶(hu)壳内溶(rong)液(ye)pH����,而酸性物质沿着(zhe)藤(teng)壶(hu)壳(ke)基底中心(xin)向(xiang)试(shi)样(yang)表(biao)面渗透,最后(hou)发(fa)生(sheng)局(ju)部(bu)腐蚀(shi)���。
3����、结(jie)论(lun)
1.钛合金在(zai)海(hai)水中(zhong)的(de)腐(fu)蚀规(gui)律(lv)受污(wu)损(sun)生(sheng)物(wu)演替(ti)规律影(ying)响(xiang)����。不同生长(zhang)阶(jie)段的(de)藤(teng)壶对TC4钛合金的腐(fu)蚀机制有所(suo)不同���,结(jie)构完整的(de)活体(ti)藤壶(hu)钙质外壳和致(zhi)密藤(teng)壶(hu)胶(jiao)能够(gou)有效隔(ge)离外界(jie)
腐(fu)蚀介质(zhi)��,死(si)亡藤壶(hu)则因壳(ke)体(ti)结(jie)构不完整与有(you)机体分解,促进(jin)腐(fu)蚀发(fa)生��。
2.污(wu)损生物(wu)附(fu)着改变海(hai)水中的(de)溶(rong)解氧向(xiang)钛(tai)合(he)金表面传(chuan)输效率(lv),从而(er)影(ying)响(xiang)钝化(hua)膜(mo)的形(xing)成与(yu)再(zai)生,同时造成(cheng)钛合(he)金表(biao)面(mian)介(jie)质性(xing)质(zhi)不(bu)均(jun)匀化(hua),促成(cheng)局(ju)部腐蚀发(fa)生���。
3.藤(teng)壶等污(wu)损生物不(bu)均(jun)匀(yun)附(fu)着形成(cheng)“封闭(bi)滞留(liu)层(ceng)”�����,导(dao)致(zhi)出现(xian)富氧区和贫氧区,构成氧浓差(cha)电(dian)池(chi)。富氧区(qu)作(zuo)为阴(yin)极(ji)接(jie)受(shou)电子发(fa)生(sheng)还(hai)原(yuan)反(fan)应(ying),贫氧区(qu)作(zuo)为(wei)阳极(ji)失(shi)去(qu)电子(zi)发生(sheng)氧化(hua)反(fan)应,促进(jin)局部腐(fu)蚀(shi)。TC4钛(tai)合金(jin)在(zai)海(hai)水、钝化(hua)膜(mo)和污损生物附(fu)着(zhe)协同(tong)作(zuo)用(yong)下,腐蚀(shi)速度(du)非线(xian)性(xing)波(bo)动变(bian)化(hua)。
参考(kao)文献(xian)
[1] 王海杰(jie), 王(wang)佳(jia), 彭(peng)欣等(deng). 钛(tai)合金在(zai) 3.5%NaCl 溶(rong)液(ye)中(zhong)的(de)腐(fu)蚀行(xing)为(wei)[J].中(zhong)国腐蚀(shi)与防(fang)护学(xue)报(bao),2015, 35(1): 75-80.
WANG H J, WANG J, PENG X, et al. Corrosion Behavior of Three Titanium Alloys in 3.5%NaCl Solution[J]. Journal of Chinese Society for Corrosion and Protection, 2015, 35(1):75-80.
[2] 訾群(qun).钛合金研(yan)究新(xin)进展(zhan)及(ji)应用现状[J].钛(tai)工(gong)业(ye)进展(zhan), 2008, 25(2): 23-27.
ZI Q. New Development of Titanium Alloy and Its Application Actuality[J]. Titanium Industry Progress, 2008, 25(2): 23-27.
[3] GORYNIN I V. Titanium Alloys for Marine Application[J]. Materials Science and Engineering, 1999, 263(2), 112-116.
[4] WAKE H, TAKAHASHI H, TAKIMOTO T, et al. Development of An Electrochemical Antifouling System for Seawater Cooling Pipelines of Power Plants Using Titanium[J].Biotechnology and Bioengineering, 2006, 95(3), 468-473.
[5] 王(wang)广夫.舰(jian)船海(hai)水(shui)管(guan)路(lu)系统防(fang)腐(fu)防污(wu)技(ji)术进展(zhan)[J].材(cai)料开(kai)发与(yu)应用(yong), 2016, 31(4): 108-112.
WANG G F. Development of Anti-corrosion and Fouling of Seawater Pipe System of Marine Ship[J]. Development and Application of Materials, 2016, 31(4): 108-112.
[6] LAQUE F L. Topics for Research in Marine Corrosion[J]. Materials Performance, 1982, 21,13-18.
[7] MARTIN W. Marine Epibiosis 1. Fouling and Antifouling - Some Basic Aspects[J]. Marine Ecology Progress Series, 1989, 58: 175-189.
[8] VINAGRE P A, SIMAS T, CRUZ E, et al. Marine Biofouling: A European Database for the Marine Renewable Energy Sector[J]. Journal of Marine Science and Engineering, 2020, 8(7):495-522.
[9] XU D K, ZHOU E Z, ZHAO Y, et al. Enhanced Resistance of 2205 Cu-bearing Duplex Stainless Steel Towards Microbiologically Influenced Corrosion by Marine Aerobic Pseudomonas Aeruginosa Biofilms[J]. Journal of Materials Science & Technology, 2018, 34,1325-1336.
[10] MACHUCA L L, BAILEY S I, GUBNER R, et al. Effect of Oxygen and Biofilms on Crevice Corrosion of UNS S31803 and UNS N08825 in Natural Seawater[J]. Corrosion Science,2013, 67, 242-255.
[11] MACHUCA L L, JEFFREY R, BAILEY S I, et al. Filtration-UV Irradiation as an Option for Mitigating the Risk of Microbiologically Influenced Corrosion of Subsea Construction Alloys in Seawater[J]. Corrosion Science, 2014, 79, 89-99.
[12] XU D K, LI Y C, GU T Y. Mechanistic Modeling of Bio-corrosion Caused by Biofilms of Sulfate Reducing Bacteria and Acid Producing Bacteria[J]. Bioelectrochemistry, 2016, 110,52-58.
[13] LI H B, ZHOU E Z, REN Y B, et al. Investigation of Microbiologically Influenced Corrosion of High Nitrogen Nickel-free Stainless Steel by Pseudomonas Aeruginosa[J].Corrosion Science, 2016, 111, 811-821.
[14] AL-MUHANNA K, HABIB K. Marine Biofouling of Different Alloys Exposed to Continuous Flowing Fresh Seawater by Electrochemical Impedance Spectroscopy[J]. Journal of Saudi Chemical Society, 2016, 20(4): 391-396.
[15] LI Z, WANG J, DONG Y Z, et al. Synergistic Effect of Chloride Ion and Shewanella Algae Accelerates the Corrosion of Ti-6Al-4V Alloy[J]. Journal of Materials Science & Technology,2021, 71, 177-185.
[16] 钱鸿昌(chang).高(gao)盐(yan)环境典型(xing)钢(gang)铁材料(liao)嗜盐古(gu)菌微生物(wu)腐蚀行为机理研(yan)究(jiu)[D].北京(jing):北京科(ke)技(ji)大(da)学(xue), 2019.
QIAN H C. Study on Microbiologically Influenced Corrosion Behavior and Mechanism of Typical Steel and Iron Materials by Halophilic Archaea in High Salinity Environment[D].Beijing: University of Science and Technology Beijing, 2019.
[17] BAUTISTA B E T, WIKIEL A J, DATENKO I, et al. Influence of Extracellular Polymeric Substances (EPS) from Pseudomonas NCIMB 2021 on the Corrosion Behavior of 70Cu-30NiAlloy in Seawater[J]. Journal of Electroanalytical Chemistry, 2015, 737, 184-197.
[18] DUAN J Z, WU S R, ZHANG X J, et al. Corrosion of Carbon Steel Influenced by Anaerobic Biofilm in Natural Seawater[J], Electrochimica Acta, 2008, 54, 22-28.
[19] HAMZAH E, HUSSAIN M Z, Ibrahim Z, et al. Influence of Pseudomonas Aeruginosa Bacteria on Corrosion Resistance of 304 Stainless Steel[J]. Corrosion Engineering Science Technology, 2013, 48, 116-120.
[20] LI S L, QU Q, LI L, et al. Bacillus Cereus S-EPS as a Dual Bio-functional Corrosion and Scale Inhibitor in Artificial Seawater[J]. Water Research, 2019, 166, 1-11.
[21] Liu T, GUO Z W, ZENG Z S, et al. Marine Bacteria Provide Lasting Anticorrosion Activity for Steel Via Biofilm-induced Mineralization[J]. ACS Applied Materials Interfaces, 2018, 10,40317-40327.
[22] 马(ma)士德,郭为民(min),刘欣(xin),等.工(gong)业纯钛(TA2)在南海(hai)三亚(ya)海洋(yang)环(huan)境(jing)试(shi)验站海(hai)水全浸(jin)的(de)生(sheng)物(wu)污(wu)损(sun)与腐(fu)蚀[J]. 海洋科学, 2018, 42(10): 23-30.
MA S D, GUO W M, LIU X, et al. Biofouling and Corrosion Analyses of Industrial Pure Titanium (TA2) Immersed in Seawater at Sanya Marine Environmental Test Station in South China Sea[J]. Marine Sciences, 2018, 42(10): 23-30.
[23] BLACKWOOD D J, LIM C S, TEO S L M, et al. Macrofouling Induced Localized Corrosion of Stainless Steel in Singapore Seawater[J]. Corrosion Science, 2017, 129, 152-160.
[24] DE BRITO L V R, COUTINHO R, CAVALCANTI E H, et al. The Influence of Macrofouling on the Corrosion Behavior of API 5L X65 Carbon Steel[J]. Biofouling, 2007, 193-201.
[25] BLACKWOOD D J, LIM C S, TEO S L M. Influence of Fouling on the Efficiency of Sacrificial Anodes in Providing Cathodic Protection in Southeast Asian Tropical Seawater[J].Biofouling, 2010, 26, 779-785.
[26] NEVILLE A, HODGKIESS T. Localised Effects of Macrofouling Species on Electrochemical Corrosion of Corrosion Resistant Alloys [J]. British Corrosion Journal, 2013,35, 54-59.
[27] NEVILLE A, HODGKIESS T. Comparative Study of Stainless Steel and Related Alloy Corrosion in Natural Sea Water[J]. British Corrosion Journal, 1988, 33(2), 111-120.
[28] DEXTER S C. Biofouling and Biocorrosion[J]. Bulletin of Electrochemistry, 1996, 12, 1-7.
[29] 严(yan)涛,张(zhang)慧(hui),李韵(yun)秋(qiu),等.污损(sun)性管栖多毛类生(sheng)态(tai)特(te)点(dian)及研(yan)究(jiu)展(zhan)望(wang)[J]. 生(sheng)态学报, 2014, 34(21):6049-6057.
YAN T, ZHANG H, LI Y Q, et al. An Overview of Fouling Sedentary Polychaetes[J]. Acta Ecologica Sinica, 2014, 34(21): 6049-6057.
[30] 李(li)争显,王(wang)浩(hao)楠(nan),赵文.钛合(he)金(jin)表(biao)面海(hai)生(sheng)物(wu)污(wu)损及(ji)防护(hu)技(ji)术的(de)研究(jiu)现(xian)状和(he)发展(zhan)趋势[J].钛工(gong)业进展, 2015, 32(6): 1-7.
LI Z X, WANG H N, ZHAO W. Current Research Situation and Development Trend of the Biofouling and Antifouling Technology on Titanium Alloy[J]. Titanium Industry Progress, 2015, 32(6): 1-7.
[31] RAMAN S, KUMAR R. Interfacial Morphology and Nanomechanics of Cement of the Barnacle, Amphibalanus Reticulatus on Metallic and Non-metallic Substrata[J]. Biofouling,2011, 27(6): 569-577.
[32] LI C, WANG G, CHEN K Y, et al. Mechanical Analysis of A Scraping Method to Remove Attached Barnacles[J]. Journal of Marine Science and Engineering, 2020, 8(3): 150-164.
[33] CRISP D J, BOURGET E. Growth in Barnacles[J]. Advance in Marine Biology, 1985, 22:199-244.
[34] 铁(tie)镝,刘贵(gui)昌(chang),刘(liu)晓军,等(deng).环境温(wen)度对(dui)东(dong)方(fang)小藤(teng)壶(hu)(Chthamalus challengeri)生命(ming)表征的(de)影(ying)[J].海(hai)洋环境(jing)科学, 2010, 29(2): 191-195.
TIE D, LIU G C, LIU X J, et al. Influence of Environmental Temperature on Vital Status of Barnacle: Chthamalus Challengeri[J]. Marine Environmental Science, 2010, 29(2): 191-195.
[35] SOUTHWARD A J. On the Behavior of Barnacles III. Further Observations on the Influence of Temperature and Age on Cirral Activity [J]. Journal of the Marine Biological Association of the United Kingdom, 1957, 36(2): 323-334.
[36] 李友炽,王(wang)贵(gui),吴敬(jing)权(quan),等(deng).海(hai)洋(yang)污损(sun)生(sheng)物(wu)藤壶(hu)生(sheng)长过(guo)程及(ji)附着(zhe)强度研究(jiu)[J].海(hai)洋科(ke)学(xue), 2023,47(8): 60-67.
LI Y C, WANG G, WU J Q, et al. Study on the Growth Process and Attachment Strength of Marine Fouling Barnacles[J]. Marine Sciences, 2023, 47(8): 60-67.
[37] WENDT D E, KOWALKE G L, KIM J, et al. Factors that Influence Elastomeric Coating Performance: the Effect of Coating Thickness on Basal Plate Morphology, Growth and Critical Removal Stress of the Barnacle Balanus Amphitrite[J]. Biofouling, 2006, 22(1): 1-9.
[38] 马(ma)士德,谢肖勃(bo),黄修(xiu)明,等.藤壶附(fu)着(zhe)对(dui)海(hai)水(shui)中(zhong)金属腐(fu)蚀的影响(xiang)[J].中国(guo)腐(fu)蚀与(yu)防护(hu)学报,1995, 15(1): 74-78.
MA S D, XIE X B, HUANG X M, et al. The Effect of Barnacle Adhesion on Metal Corrosion in Seawater[J]. Journal of Chinese Society for Corrosion and Protection, 1995, 15(1): 74-78.
[39] ZHENG X W, ZHUANG X, LEI Y H, et al. Corrosion Behavior of the Ti-6Al-4V Alloy in Sulfate-Reducing Bacteria Solution[J]. Coatings, 2019, 10(1), 24.
[40] DENG P C, SHANGGUAN J Y, HU J Z, et al. Effect of Barnacles on the Corrosion Behavior of 304 Stainless Steel[J]. Metals, 2023, 13, 1649-1658.
[41] VENKATESAN R, MURTHY P S. Macro-fouling Control in Power Plants[M]. Berlin, Heidelberg: Springer, 2008.
[42] LEWIS J A. Marine Biofouling and Its Prevention on Underwater Surfaces[J]. Materials Forum, 1998, 22: 41-61.
[43] 马士(shi)德(de). 金(jin)属/海(hai)水(shui)界(jie)面(mian)两(liang)个(ge)主(zhu)要过程的(de)关(guan)系(xi)[J].海(hai)洋湖沼通(tong)报(bao), 1979, 02: 85-89.
MA S D. The Relationship of Two Main Process on Metal/Sea Water[J]. Transactions of Oceanology and Limnology, 1979, 02: 85-89.
[44] SANGEETHA R, KUMAR R, DOBLE M, et al. Barnacle Cement: An Etchant for Stainless Steel 316L[J]. Colloids and Surfaces B: Biointerfaces, 2010, 79(2), 524-530.
[45] EASHWAR M, SUBRAMANIAN G, CHANDRASEKARAN P, et al. Mechanism for Barnacle-Induced Crevice Corrosion in Stainless Steel[J]. Corrosion, 1992, 48(7), 608-612.
相关链接




















